Chemistry 161 General Chemistry 1 Stoichiometry of Chemical





























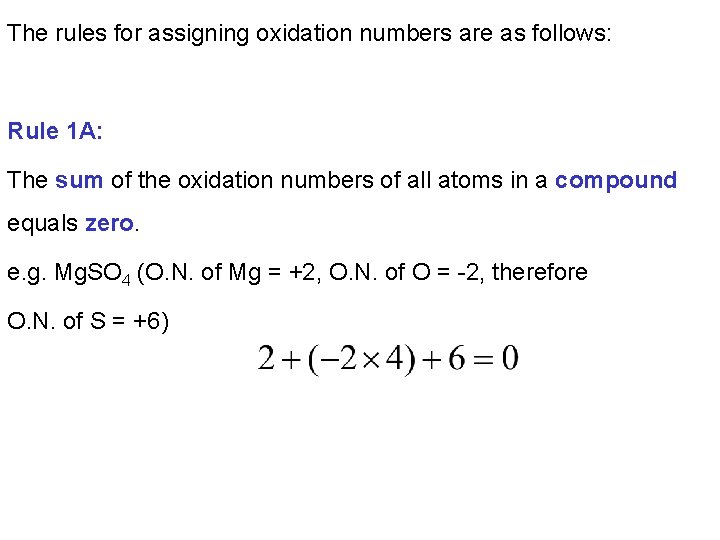


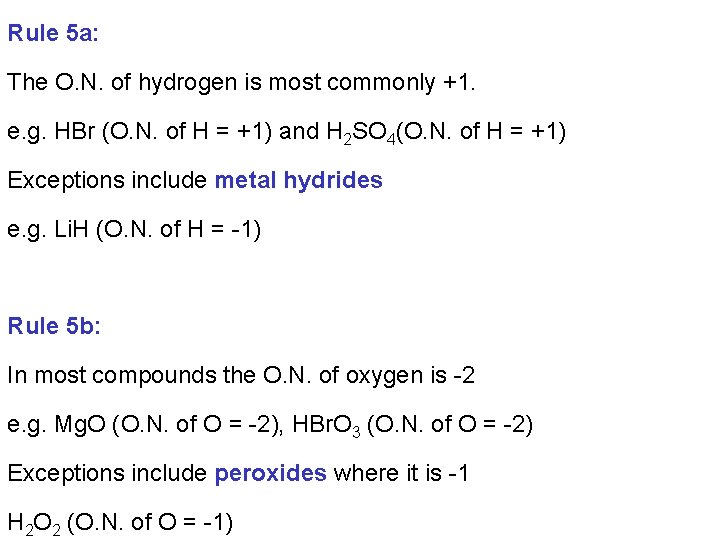



- Slides: 99

Chemistry& 161 General Chemistry 1

Stoichiometry of Chemical Reactions 4. 1 Writing and Balancing Chemical Equations 4. 2 Classifying Chemical Reactions 4. 3 Reaction Stoichiometry 4. 4 Reaction Yields 4. 5 Quantitative Chemical Analysis

Stoichiometry of Chemical Reactions 4. 1 Writing and Balancing Chemical Equations

Thinking of the language of chemistry we might say: • Element symbols are like letters • Chemical Formulas are like words • Chemical Equations are like sentences What do we mean by a “chemical equation”?

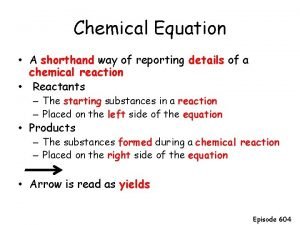
We can define a chemical equation as: “a statement in formulas and atomic symbols that expresses the identities and quantities of substances involved in a chemical or physical change” An equation that correctly describes the amounts of substances is described as being “balanced”.

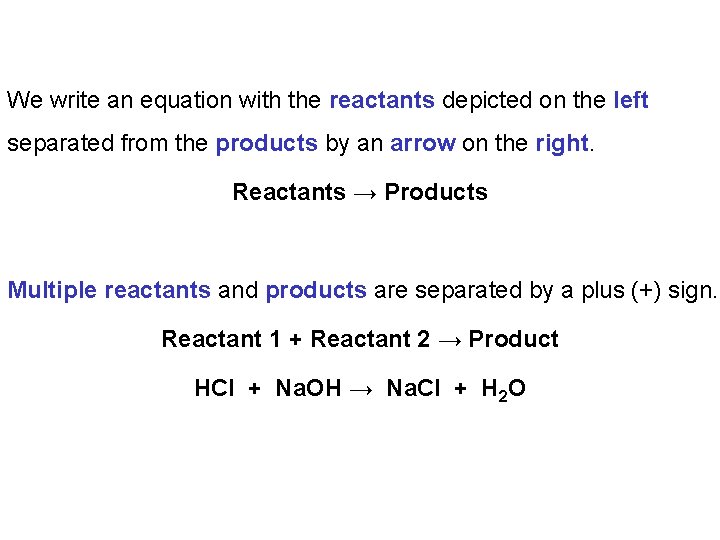
We write an equation with the reactants depicted on the left separated from the products by an arrow on the right. Reactants → Products Multiple reactants and products are separated by a plus (+) sign. Reactant 1 + Reactant 2 → Product HCl + Na. OH → Na. Cl + H 2 O

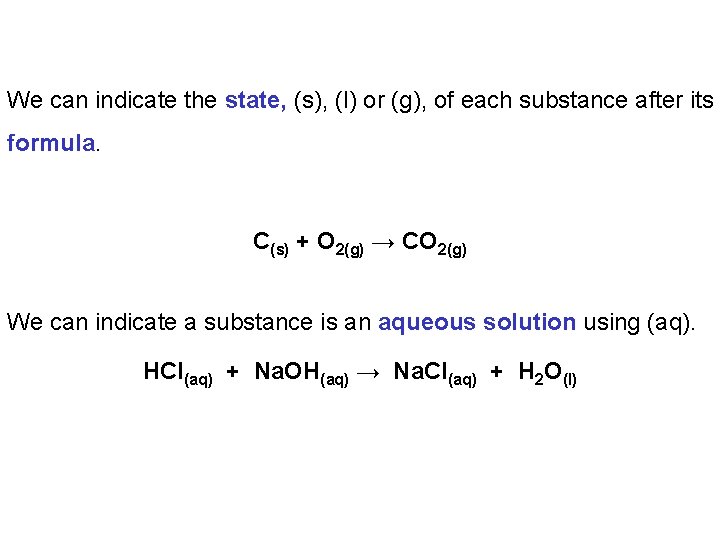
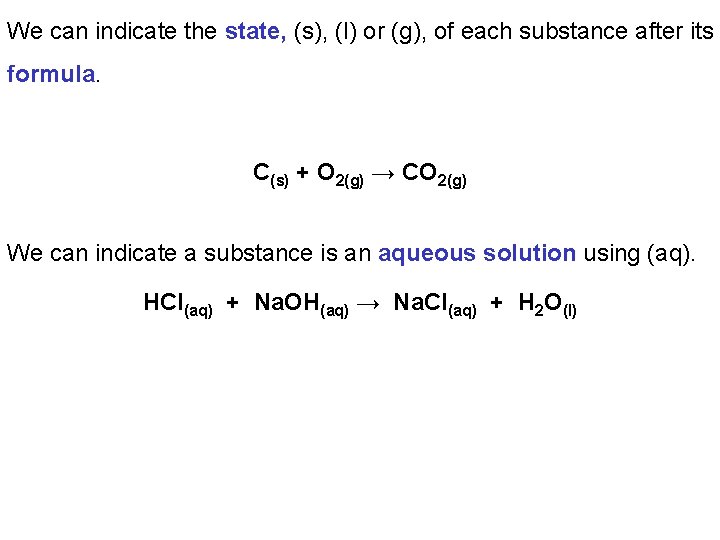
We can indicate the state, (s), (l) or (g), of each substance after its formula. C(s) + O 2(g) → CO 2(g) We can indicate a substance is an aqueous solution using (aq). HCl(aq) + Na. OH(aq) → Na. Cl(aq) + H 2 O(l)

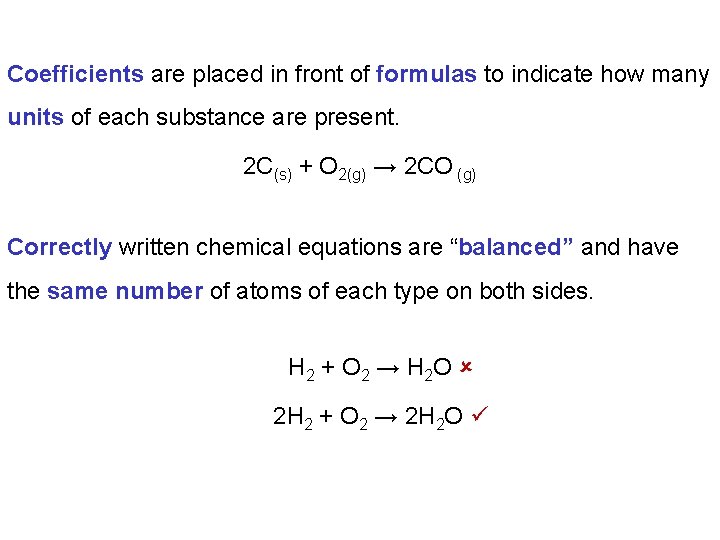
Coefficients are placed in front of formulas to indicate how many units of each substance are present. 2 C(s) + O 2(g) → 2 CO (g) Correctly written chemical equations are “balanced” and have the same number of atoms of each type on both sides. H 2 + O 2 → H 2 O 2 H 2 + O 2 → 2 H 2 O

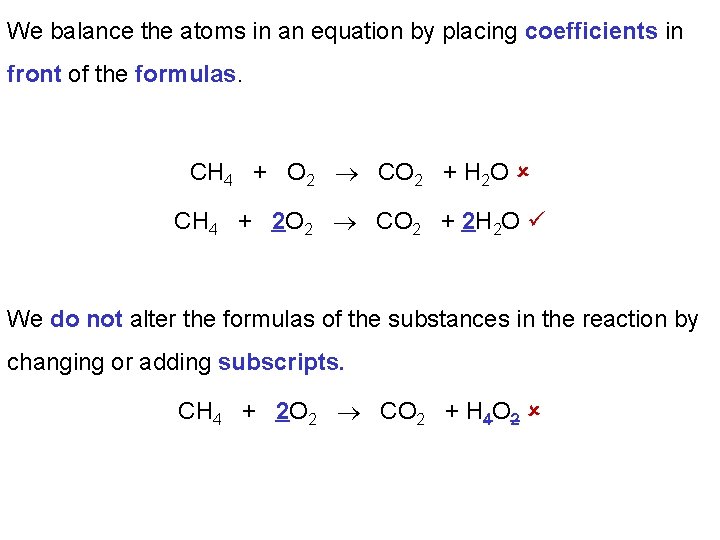
We balance the atoms in an equation by placing coefficients in front of the formulas. CH 4 + O 2 CO 2 + H 2 O CH 4 + 2 O 2 CO 2 + 2 H 2 O We do not alter the formulas of the substances in the reaction by changing or adding subscripts. CH 4 + 2 O 2 CO 2 + H 4 O 2

We can’t randomly add substances in order to balance the equation. CH 4 + O 2 CO 2 + H 2 O CH 4 + O 2 + 2 O CO 2 + H 2 O 2 CH 4 + 2 O 2 CO 2 + 2 H 2 O We try to make the coefficients the smallest whole number possible. CH 4 + 2 O 2 CO 2 + 2 H 2 O 2 CH 4 + 4 O 2 2 CO 2 + 4 H 2 O

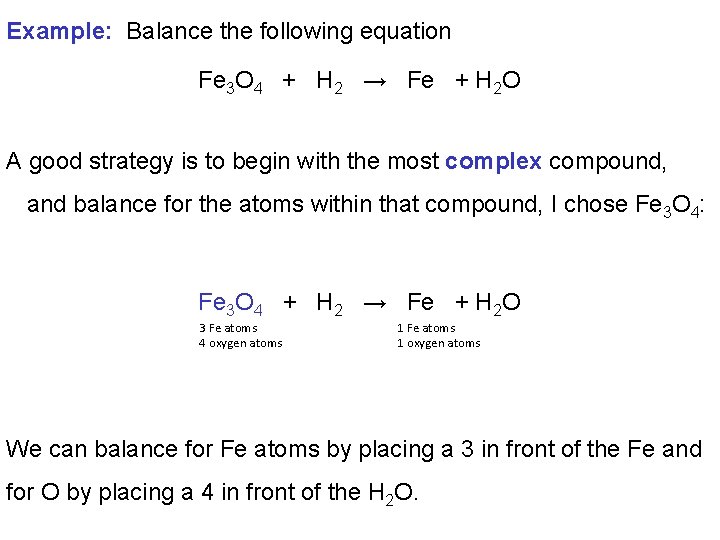
Example: Balance the following equation Fe 3 O 4 + H 2 → Fe + H 2 O A good strategy is to begin with the most complex compound, and balance for the atoms within that compound, I chose Fe 3 O 4: Fe 3 O 4 + H 2 → Fe + H 2 O 3 Fe atoms 4 oxygen atoms 1 Fe atoms 1 oxygen atoms We can balance for Fe atoms by placing a 3 in front of the Fe and for O by placing a 4 in front of the H 2 O.

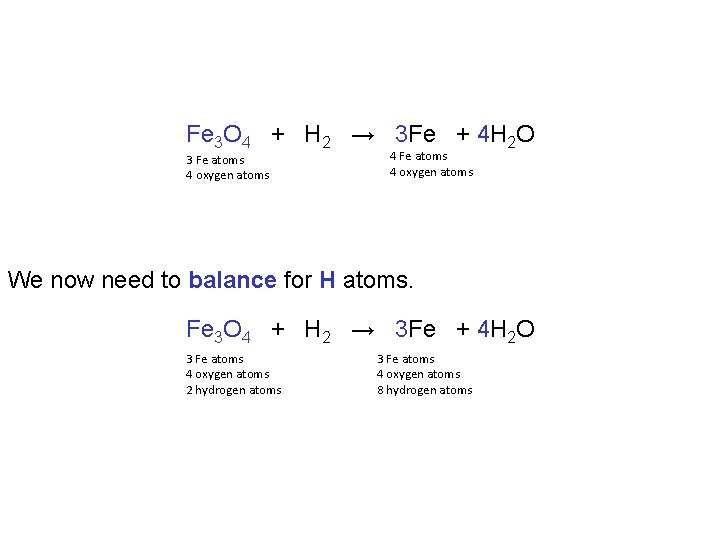
Fe 3 O 4 + H 2 → 3 Fe + 4 H 2 O 3 Fe atoms 4 oxygen atoms 4 Fe atoms 4 oxygen atoms We now need to balance for H atoms. Fe 3 O 4 + H 2 → 3 Fe + 4 H 2 O 3 Fe atoms 4 oxygen atoms 2 hydrogen atoms 3 Fe atoms 4 oxygen atoms 8 hydrogen atoms

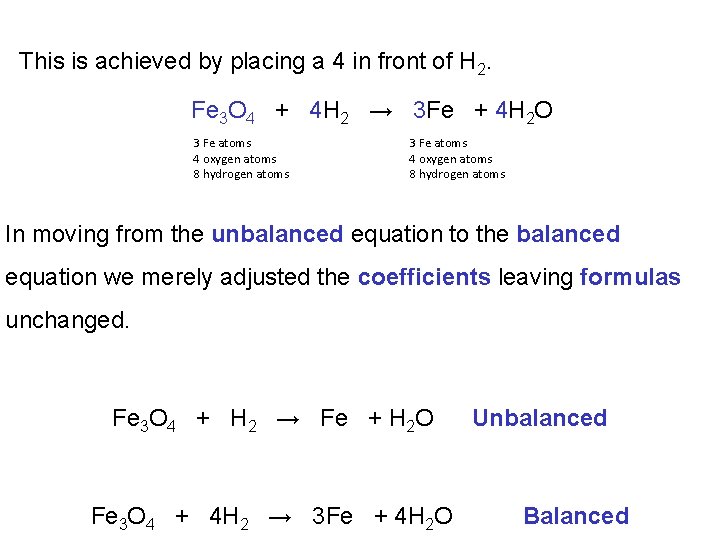
This is achieved by placing a 4 in front of H 2. Fe 3 O 4 + 4 H 2 → 3 Fe + 4 H 2 O 3 Fe atoms 4 oxygen atoms 8 hydrogen atoms In moving from the unbalanced equation to the balanced equation we merely adjusted the coefficients leaving formulas unchanged. Fe 3 O 4 + H 2 → Fe + H 2 O Fe 3 O 4 + 4 H 2 → 3 Fe + 4 H 2 O Unbalanced Balanced

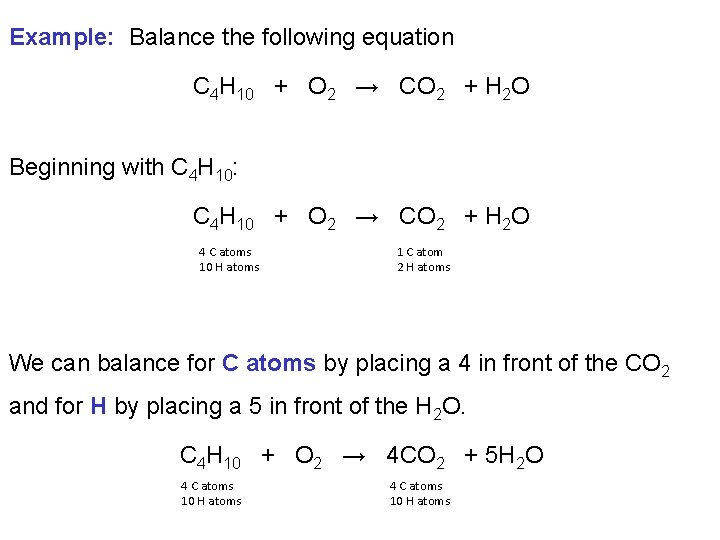
Example: Balance the following equation C 4 H 10 + O 2 → CO 2 + H 2 O Beginning with C 4 H 10: C 4 H 10 + O 2 → CO 2 + H 2 O 4 C atoms 10 H atoms 1 C atom 2 H atoms We can balance for C atoms by placing a 4 in front of the CO 2 and for H by placing a 5 in front of the H 2 O. C 4 H 10 + O 2 → 4 CO 2 + 5 H 2 O 4 C atoms 10 H atoms

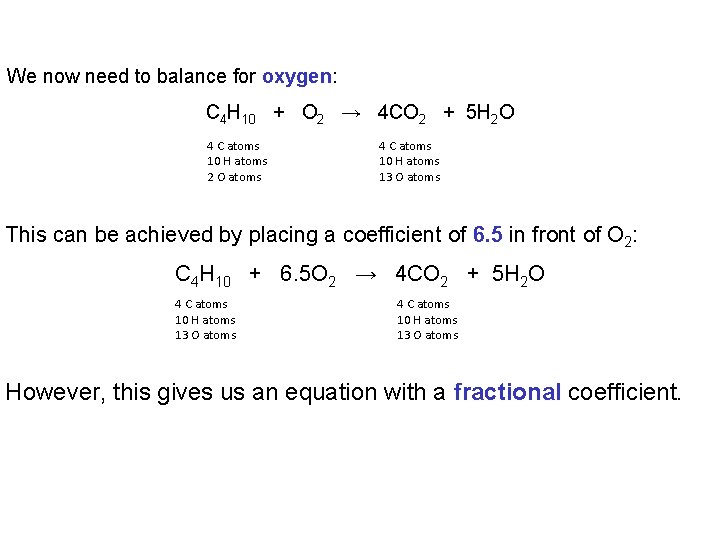
We now need to balance for oxygen: C 4 H 10 + O 2 → 4 CO 2 + 5 H 2 O 4 C atoms 10 H atoms 2 O atoms 4 C atoms 10 H atoms 13 O atoms This can be achieved by placing a coefficient of 6. 5 in front of O 2: C 4 H 10 + 6. 5 O 2 → 4 CO 2 + 5 H 2 O 4 C atoms 10 H atoms 13 O atoms However, this gives us an equation with a fractional coefficient.

To remove the fraction we multiply all the coefficients by 2: 2 C 4 H 10 + 13 O 2 → 8 CO 2 + 10 H 2 O 8 C atoms 20 H atoms 26 O atoms Frequently, our initial attempt to balance an equation will have a fractional coefficient if the equation contains a diatomic element.

We can indicate the state, (s), (l) or (g), of each substance after its formula. C(s) + O 2(g) → CO 2(g) We can indicate a substance is an aqueous solution using (aq). HCl(aq) + Na. OH(aq) → Na. Cl(aq) + H 2 O(l)

Stoichiometry of Chemical Reactions 4. 1 Writing and Balancing Chemical Equations are balanced by placing coefficients in front of one or more chemical formulas so that there is an equal number of each atom types in both the products and reactants. The coefficients used should be the simplest set of whole numbers that balance the equation. The symbols (s), (l), (g) and (aq) can be used to indicate if a substance is present as a solid, liquid, gas or aqueous solution, respectively.

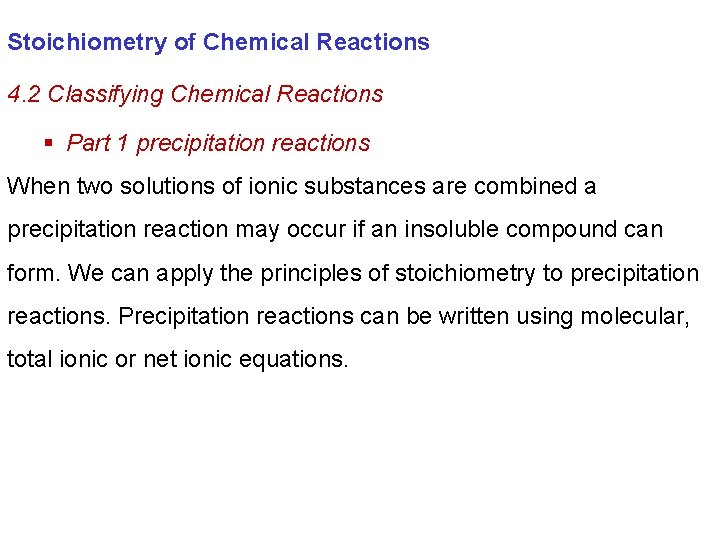
Stoichiometry of Chemical Reactions 4. 2 Classifying Chemical Reactions § Part 1 precipitation reactions

What happens when an ionic compound dissolves in water? the attractive forces between water molecules and the ions are greater than the forces between oppositely charged ions and the ionic compound dissolves.

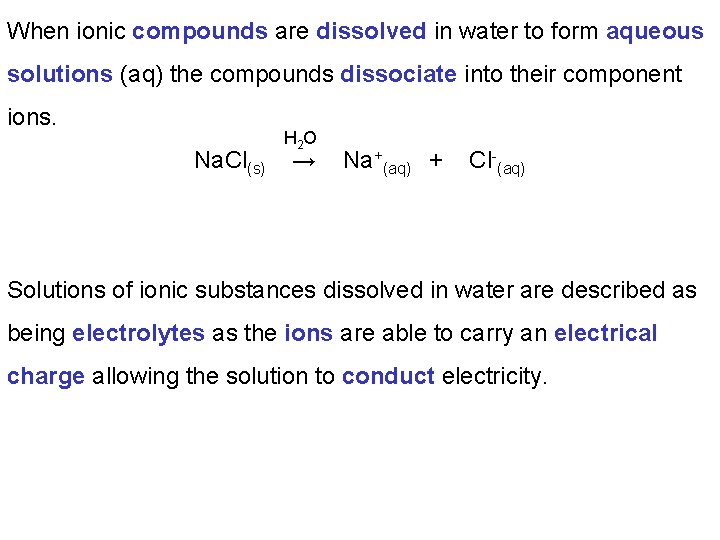
When ionic compounds are dissolved in water to form aqueous solutions (aq) the compounds dissociate into their component ions. Na. Cl(s) H 2 O → Na+(aq) + Cl-(aq) Solutions of ionic substances dissolved in water are described as being electrolytes as the ions are able to carry an electrical charge allowing the solution to conduct electricity.

Consider an aqueous Na. Cl solution. Distilled water Na. Cl(s) Na. Cl(aq) In the aqueous solution the Na+ ions move towards the negative electrode and the Cl- ions move towards the positive electrode conducting the electric current.

Some covalent compounds are also soluble in water. H 2 O CH 3 CH 2 OH(l) → CH 3 CH 2 OH(aq) Many of these substances do not dissociate into ions but remain as intact neutral molecules. Aqueous solutions of covalent molecules that do not conduct electricity contain solutes described as being non-electrolytes.

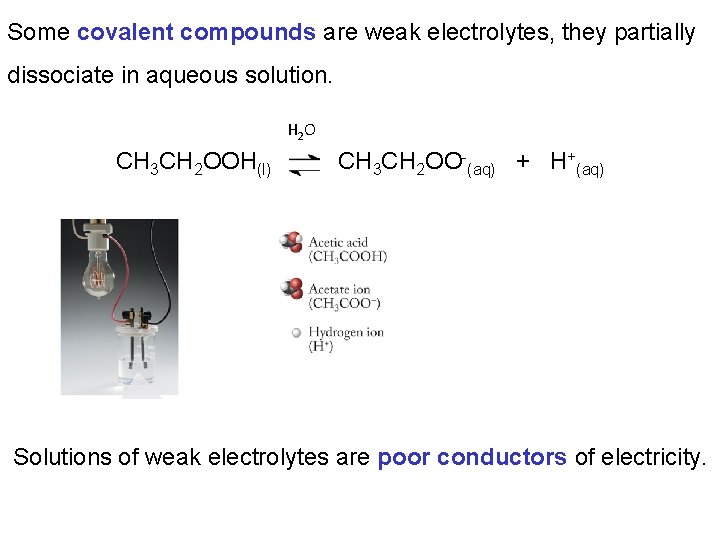
Some covalent compounds are weak electrolytes, they partially dissociate in aqueous solution. H 2 O CH 3 CH 2 OOH(l) CH 3 CH 2 OO-(aq) + H+(aq) Solutions of weak electrolytes are poor conductors of electricity.

Some ionic compounds are readily soluble in water many of these are observed in the oceans. Other ionic compounds are insoluble in water, these tend to be present in the Earth’s crust, rather than the oceans. We will consider a compound to be insoluble if its solubility is less 0. 01 mol/L Sodium Chloride (aka sea salt)

A saturated solution is one that contains the maximum amount of solute. Filtering this solution would yield a saturated solution

Sometimes it possible to create a supersaturated solution containing more solute than we would predict based on its solubility. These types of solutions can be formed by very slow cooling or evaporation of solvent. A slight disturbance will cause the excess solute to crystallize.

Ionic compounds containing the following ions tend to be soluble: Group 1 A and ammonium ions. Nitrates (NO 3 -) Acetates (CH 3 COO-) Halide ions (group 7 A) except those of Ag+, Pb 2+, Cu+ and Hg 2+. Sulfates (SO 42 -) except those of Ca 2+, Sr 2+, Ba 2+, Ag+ and Pb 2+

Ionic compounds containing the following ions tend to be insoluble: Hydroxides (OH-) except those of group 1 A and Ca 2+, Sr 2+, Ba 2+ Carbonates (CO 32 -) except those of group 1 A and NH 4+ Phosphates (PO 43 -) except those of group 1 A and NH 4+ Sulfides (S 2 -)except those of group 1 A, Ca 2+, Sr 2+, Ba 2+ and NH 4+

When two solutions of ionic compounds are mixed a solid product or precipitate may form: e. g. Sr. Cl 2(aq) + Na 2 CO 3(aq) → Sr. CO 3(s) + 2 Na. Cl(aq) Solution 1: Sr. Cl 2(aq) → Sr 2+(aq) + 2 Cl-(aq) Solution 2: Na 2 CO 3(aq) → 2 Na+(aq) + CO 32 -(aq)

How can we predict if a precipitate will form when solutions of ionic compounds are combined? e. g. Solution 1: Sr. Cl 2(aq) → Sr 2+(aq) + 2 Cl-(aq) Solution 2: Na 2 CO 3(aq) → 2 Na+(aq) + CO 32 -(aq) Consideration of the “solubility rules” indicates solid Sr. CO 3 will form when these two solutions are combined.

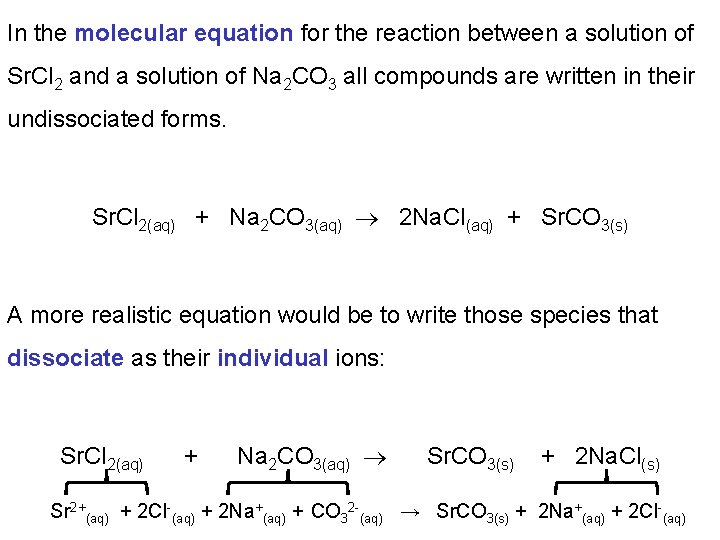
In the molecular equation for the reaction between a solution of Sr. Cl 2 and a solution of Na 2 CO 3 all compounds are written in their undissociated forms. Sr. Cl 2(aq) + Na 2 CO 3(aq) 2 Na. Cl(aq) + Sr. CO 3(s) A more realistic equation would be to write those species that dissociate as their individual ions: Sr. Cl 2(aq) + Na 2 CO 3(aq) Sr. CO 3(s) + 2 Na. Cl(s) Sr 2+(aq) + 2 Cl-(aq) + 2 Na+(aq) + CO 32 -(aq) → Sr. CO 3(s) + 2 Na+(aq) + 2 Cl-(aq)

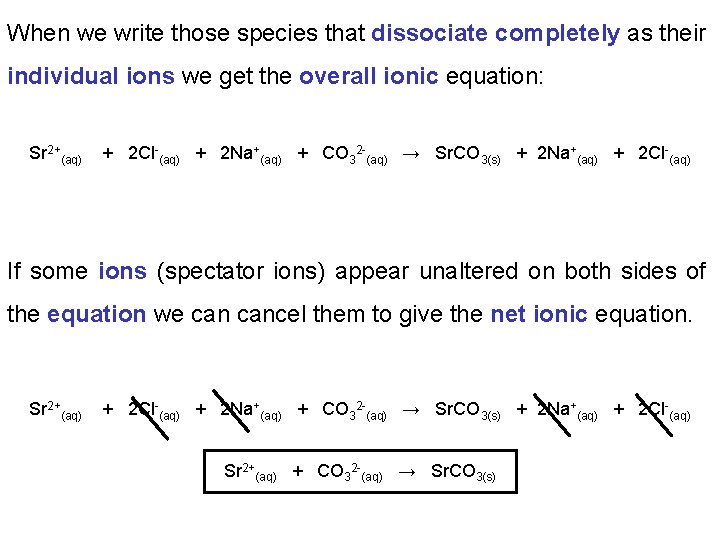
When we write those species that dissociate completely as their individual ions we get the overall ionic equation: Sr 2+(aq) + 2 Cl-(aq) + 2 Na+(aq) + CO 32 -(aq) → Sr. CO 3(s) + 2 Na+(aq) + 2 Cl-(aq) If some ions (spectator ions) appear unaltered on both sides of the equation we cancel them to give the net ionic equation. Sr 2+(aq) + 2 Cl-(aq) + 2 Na+(aq) + CO 32 -(aq) → Sr. CO 3(s) + 2 Na+(aq) + 2 Cl-(aq) Sr 2+(aq) + CO 32 -(aq) → Sr. CO 3(s)

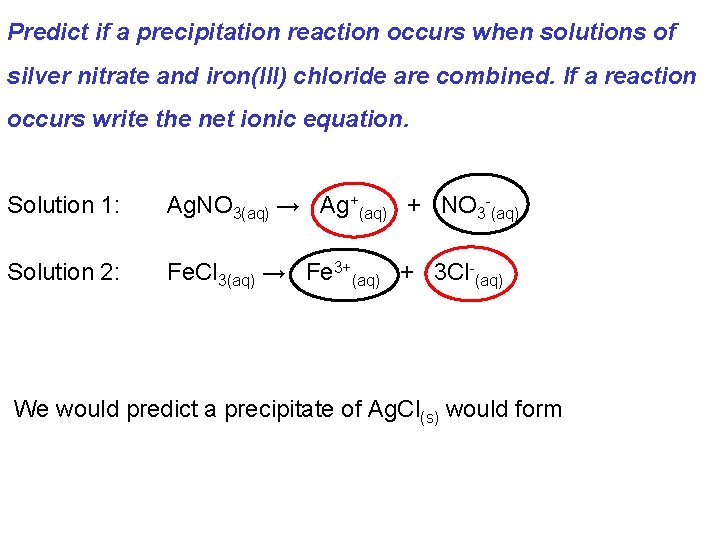
Predict if a precipitation reaction occurs when solutions of silver nitrate and iron(III) chloride are combined. If a reaction occurs write the net ionic equation. Solution 1: Ag. NO 3(aq) → Ag+(aq) + NO 3 -(aq) Solution 2: Fe. Cl 3(aq) → Fe 3+(aq) + 3 Cl-(aq) We would predict a precipitate of Ag. Cl(s) would form

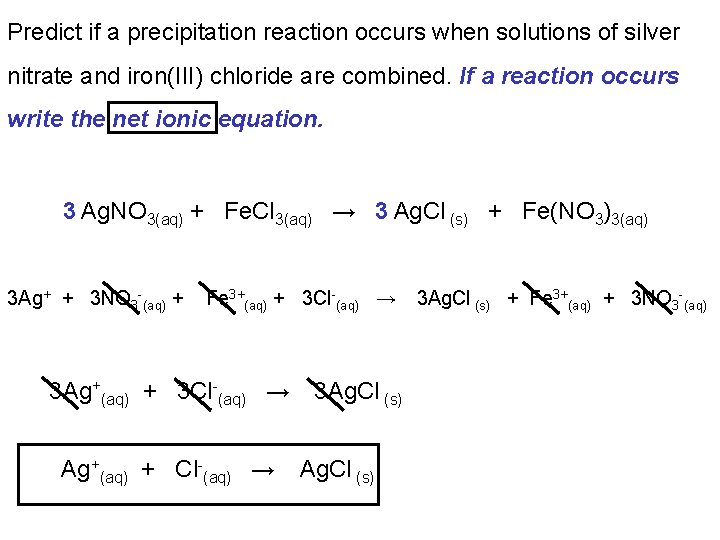
Predict if a precipitation reaction occurs when solutions of silver nitrate and iron(III) chloride are combined. If a reaction occurs write the net ionic equation. 3 Ag. NO 3(aq) + Fe. Cl 3(aq) → 3 Ag. Cl (s) + Fe(NO 3)3(aq) 3 Ag+ + 3 NO 3 -(aq) + Fe 3+(aq) + 3 Cl-(aq) → 3 Ag+(aq) + 3 Cl-(aq) → Ag+(aq) + Cl-(aq) → 3 Ag. Cl (s) + Fe 3+(aq) + 3 NO 3 -(aq)

Precipitation reaction problems may involve stoichiometry calculations and naming. e. g. Determine the mass of precipitate that forms when 25 m. L of 0. 10 mol/L silver nitrate is combined with excess iron(III) chloride.

Determine the mass of precipitate that forms when 25 m. L of 0. 10 mol/L silver nitrate is combined with excess iron(III) chloride.

Stoichiometry of Chemical Reactions 4. 2 Classifying Chemical Reactions § Part 1 precipitation reactions When two solutions of ionic substances are combined a precipitation reaction may occur if an insoluble compound can form. We can apply the principles of stoichiometry to precipitation reactions. Precipitation reactions can be written using molecular, total ionic or net ionic equations.

Stoichiometry of Chemical Reactions 4. 2 Classifying Chemical Reactions § Part 2 acid-base reactions

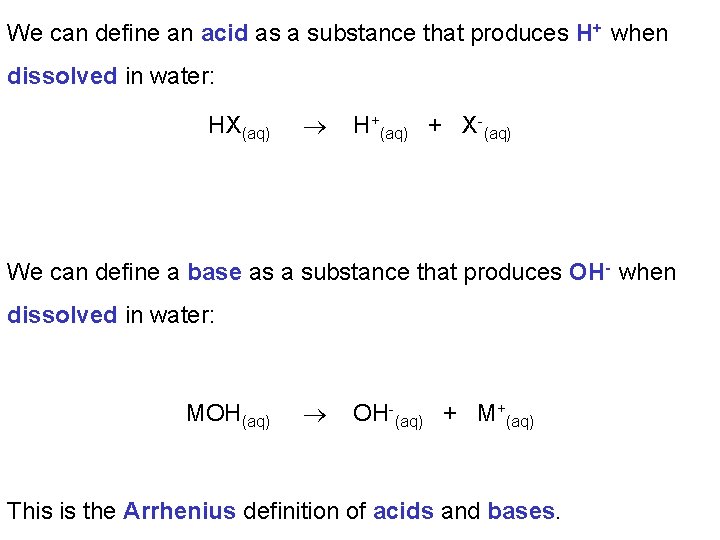
We can define an acid as a substance that produces H+ when dissolved in water: HX(aq) H+(aq) + X-(aq) We can define a base as a substance that produces OH- when dissolved in water: MOH(aq) OH-(aq) + M+(aq) This is the Arrhenius definition of acids and bases.

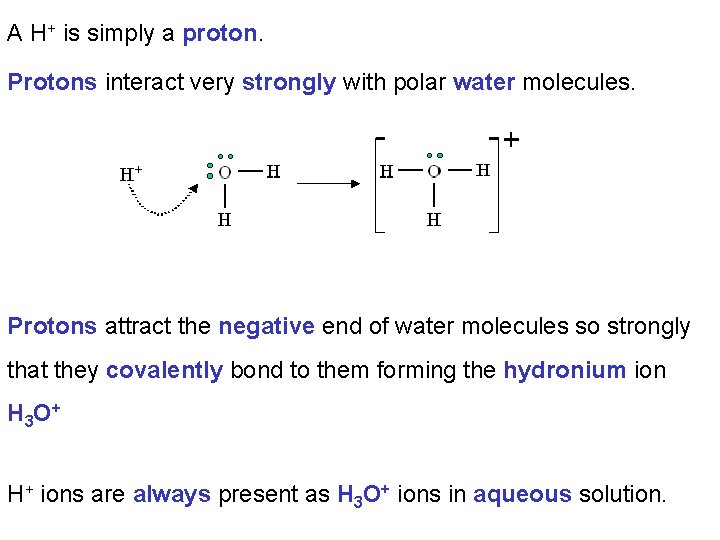
A H+ is simply a proton. Protons interact very strongly with polar water molecules. Protons attract the negative end of water molecules so strongly that they covalently bond to them forming the hydronium ion H 3 O + H+ ions are always present as H 3 O+ ions in aqueous solution.

We can now write the dissolution of an acid in water as follows: HCl(g) + H 2 O(l) Cl-(aq) + H 3 O+(aq) This clearly indicates the process that is occurring. However, you should be aware that people will frequently write H+(aq) instead of H 3 O+(aq) and assume you understand the proton is solvated.

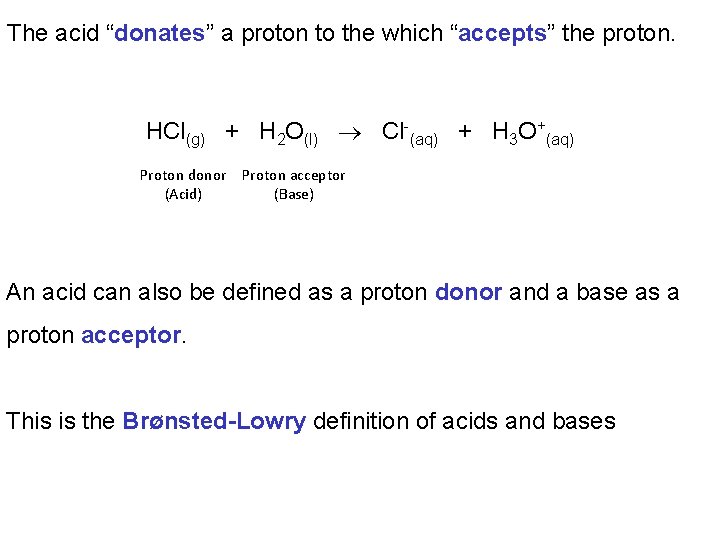
The acid “donates” a proton to the which “accepts” the proton. HCl(g) + H 2 O(l) Cl-(aq) + H 3 O+(aq) Proton donor Proton acceptor (Acid) (Base) An acid can also be defined as a proton donor and a base as a proton acceptor. This is the Brønsted-Lowry definition of acids and bases

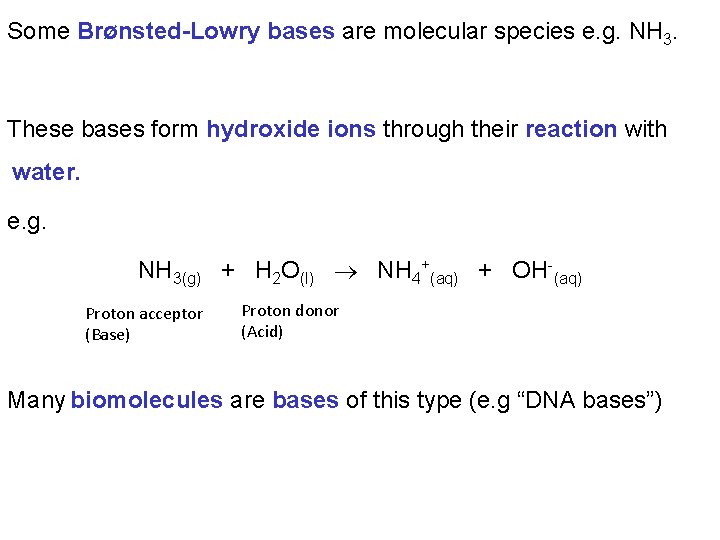
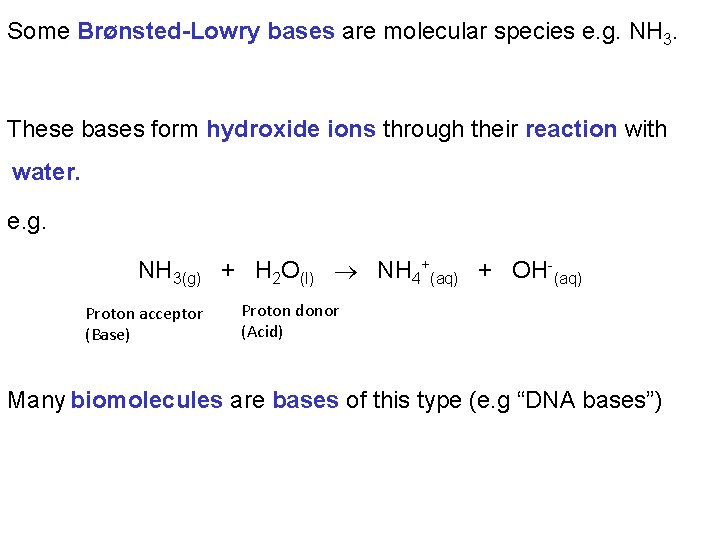
Some Brønsted-Lowry bases are molecular species e. g. NH 3. These bases form hydroxide ions through their reaction with water. e. g. NH 3(g) + H 2 O(l) NH 4+(aq) + OH-(aq) Proton acceptor (Base) Proton donor (Acid) Many biomolecules are bases of this type (e. g “DNA bases”)

Some Brønsted-Lowry bases are molecular species e. g. NH 3. These bases form hydroxide ions through their reaction with water. e. g. NH 3(g) + H 2 O(l) NH 4+(aq) + OH-(aq) Proton acceptor (Base) Proton donor (Acid) Many biomolecules are bases of this type (e. g “DNA bases”)

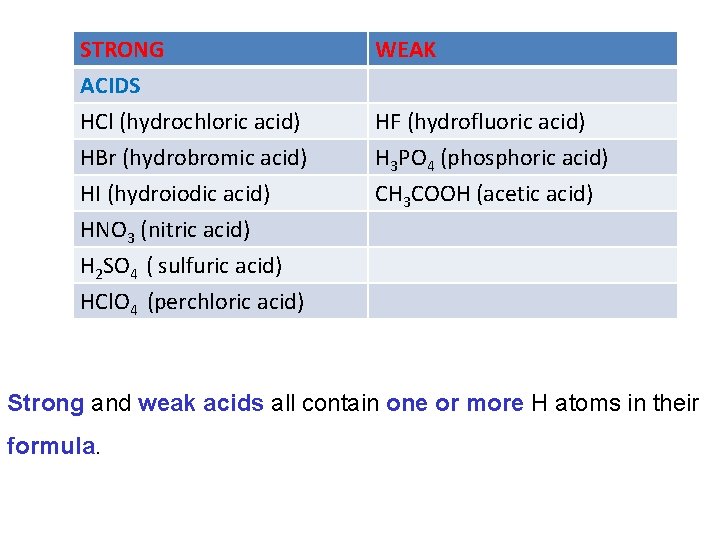
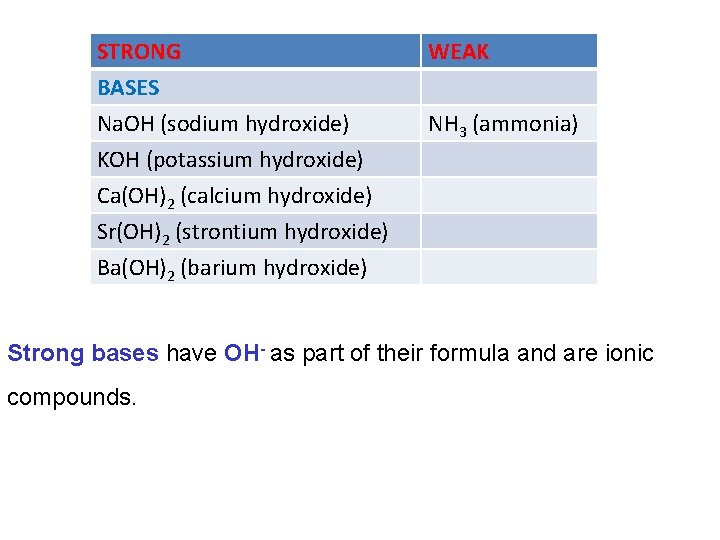
The strength of acids and bases are categorized according to the extent they dissociate into ions in aqueous solution. • Strong acids and bases dissociate completely • Weak acids and bases dissociate very little, most of their molecules stay intact. It is very important not to confuse the strength of an acid or base with its concentration.

STRONG ACIDS HCl (hydrochloric acid) HBr (hydrobromic acid) WEAK HI (hydroiodic acid) HNO 3 (nitric acid) H 2 SO 4 ( sulfuric acid) HCl. O 4 (perchloric acid) CH 3 COOH (acetic acid) HF (hydrofluoric acid) H 3 PO 4 (phosphoric acid) Strong and weak acids all contain one or more H atoms in their formula.

STRONG BASES Na. OH (sodium hydroxide) KOH (potassium hydroxide) Ca(OH)2 (calcium hydroxide) Sr(OH)2 (strontium hydroxide) Ba(OH)2 (barium hydroxide) WEAK NH 3 (ammonia) Strong bases have OH- as part of their formula and are ionic compounds.

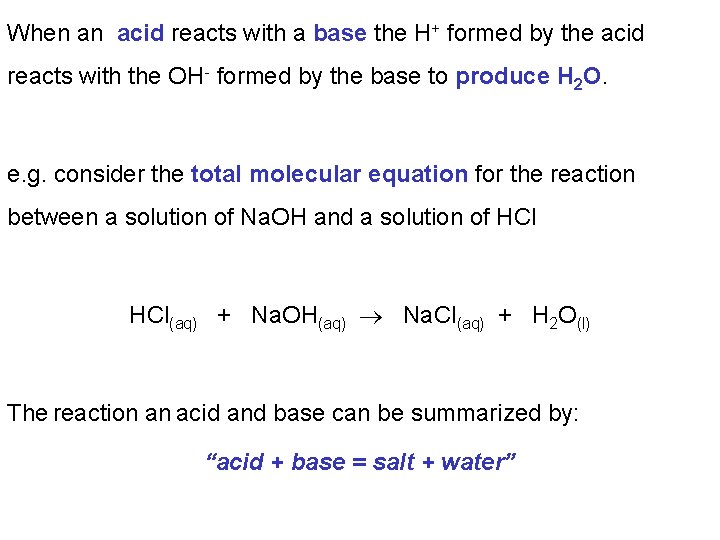
When an acid reacts with a base the H+ formed by the acid reacts with the OH- formed by the base to produce H 2 O. e. g. consider the total molecular equation for the reaction between a solution of Na. OH and a solution of HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O(l) The reaction an acid and base can be summarized by: “acid + base = salt + water”

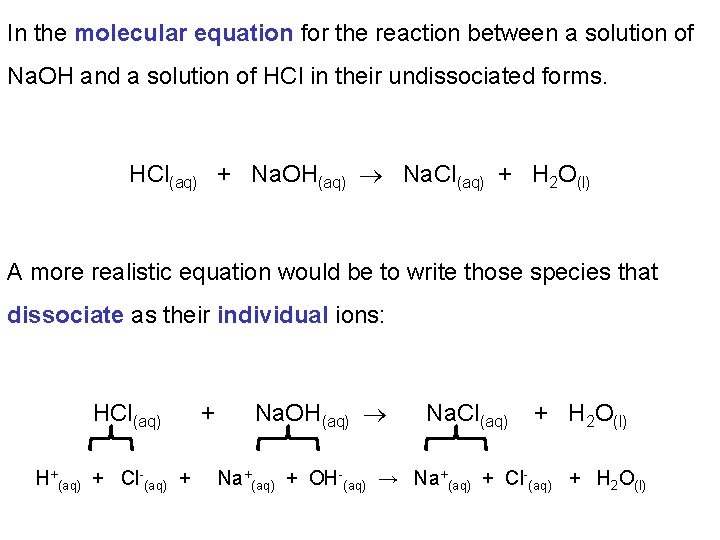
In the molecular equation for the reaction between a solution of Na. OH and a solution of HCl in their undissociated forms. HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O(l) A more realistic equation would be to write those species that dissociate as their individual ions: HCl(aq) H+(aq) + Cl-(aq) + + Na. OH(aq) Na. Cl(aq) + H 2 O(l) Na+(aq) + OH-(aq) → Na+(aq) + Cl-(aq) + H 2 O(l)

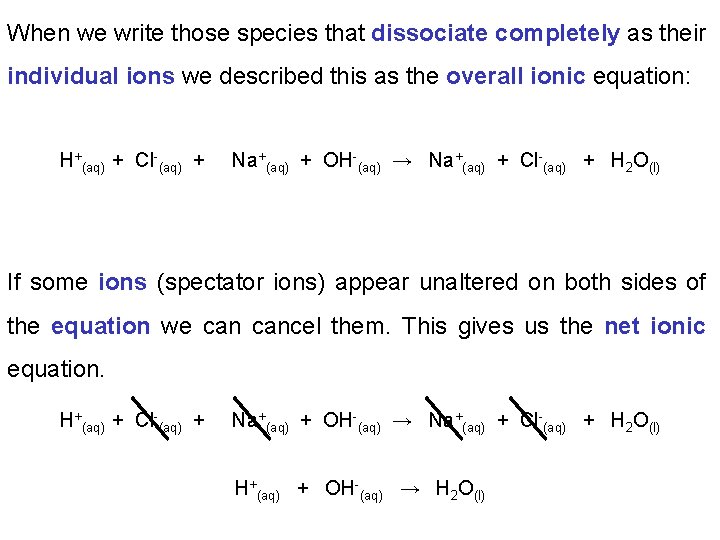
When we write those species that dissociate completely as their individual ions we described this as the overall ionic equation: H+(aq) + Cl-(aq) + Na+(aq) + OH-(aq) → Na+(aq) + Cl-(aq) + H 2 O(l) If some ions (spectator ions) appear unaltered on both sides of the equation we cancel them. This gives us the net ionic equation. H+(aq) + Cl-(aq) + Na+(aq) + OH-(aq) → Na+(aq) + Cl-(aq) + H 2 O(l) H+(aq) + OH-(aq) → H 2 O(l)

Stoichiometry of Chemical Reactions 4. 2 Classifying Chemical Reactions § Part 2 acid-base reactions Acids can be described as proton donors and bases as protons acceptors. Acids and bases react in neutralization reactions to form a salt (ionic compound) and water. Reactions involving species that dissociate to form ions maybe written as either a molecular equation, an overall ionic equation or a net ionic equation.

Stoichiometry of Chemical Reactions 4. 2 Classifying Chemical Reactions § Part 3 oxidation-reduction reactions

Another important class of reactions are oxidation-reduction reactions or redox reactions. In redox reactions electrons are transferred from one reactant to another. The losing of electrons is called oxidation. The gaining of electrons is called reduction. Oil Rig Oxidation is loss Reduction is gain

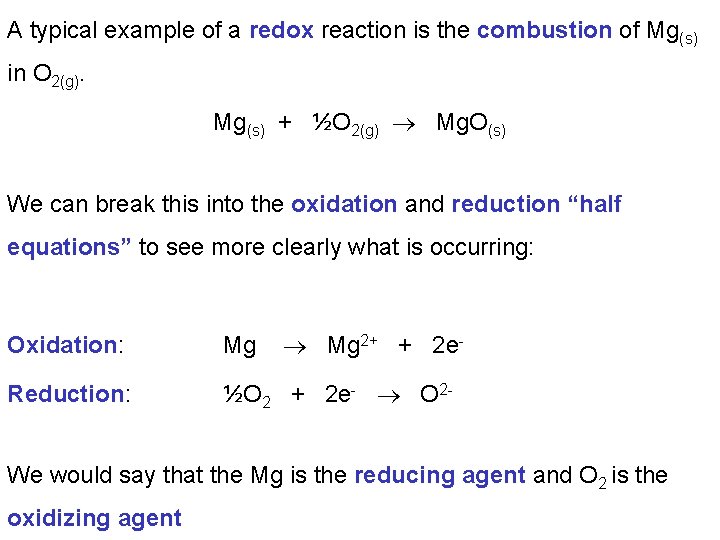
A typical example of a redox reaction is the combustion of Mg(s) in O 2(g). Mg(s) + ½O 2(g) Mg. O(s) We can break this into the oxidation and reduction “half equations” to see more clearly what is occurring: Mg 2+ + 2 e- Oxidation: Mg Reduction: ½O 2 + 2 e- O 2 - We would say that the Mg is the reducing agent and O 2 is the oxidizing agent

One of the more exciting redox reactions is thermite reaction: 2 Al(s) + Fe 2 O 3(s) 2 Fe(l) + Al 2 O 3(s)

It is important to realize that these processes occur simultaneously and cannot occur with out the other occurring as well. For this reason: The reactant that is oxidized is called the reducing agent as it reduces the other reagent. The reactant that is reduced is called the oxidizing agent as it oxidizes the other reactant.

In order to keep track of the movement of electrons a system based on “oxidation numbers” has been devised. In this system each atom type in each ion or molecule is assigned an oxidation number. How do we determine the oxidation number of an atom in a molecule or ion?
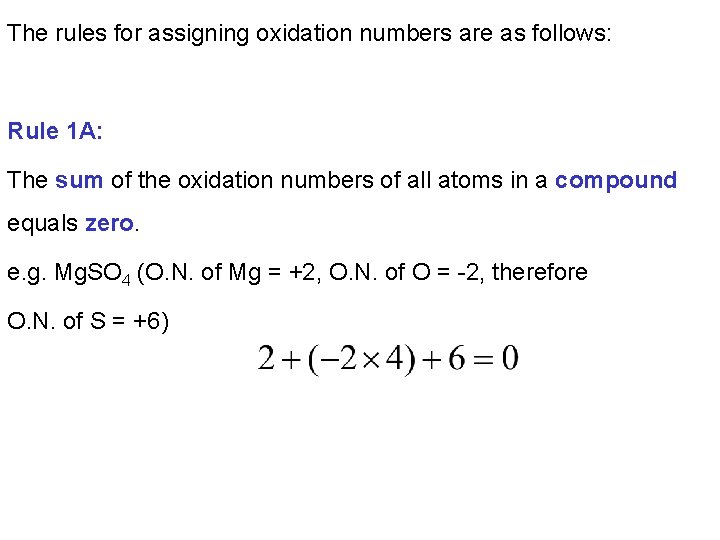
The rules for assigning oxidation numbers are as follows: Rule 1 A: The sum of the oxidation numbers of all atoms in a compound equals zero. e. g. Mg. SO 4 (O. N. of Mg = +2, O. N. of O = -2, therefore O. N. of S = +6)

Rule 1 B: The sum of the O. N. of all the atoms in a polyatomic ion is equal to the charge on the ion. e. g. HCO 3 - (O. N. of H is +1, O. N. of O is -2, and O. N. of C is +4)

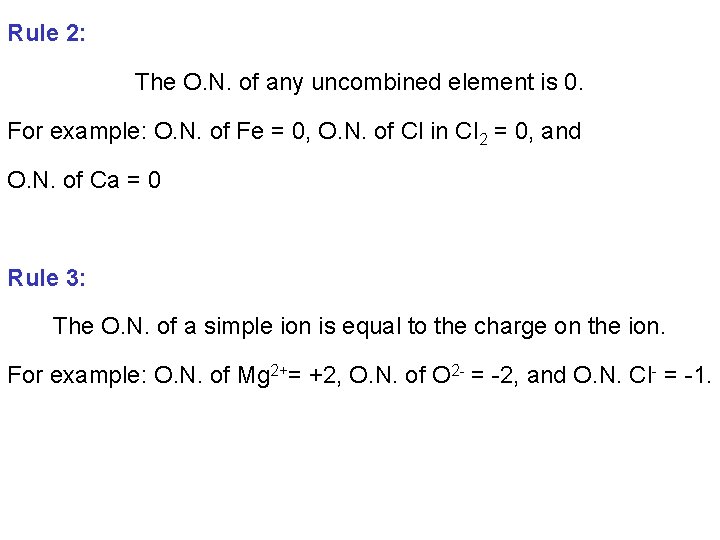
Rule 2: The O. N. of any uncombined element is 0. For example: O. N. of Fe = 0, O. N. of Cl in Cl 2 = 0, and O. N. of Ca = 0 Rule 3: The O. N. of a simple ion is equal to the charge on the ion. For example: O. N. of Mg 2+= +2, O. N. of O 2 - = -2, and O. N. Cl- = -1.

Rule 4: In compounds containing fluorine and one or more other element the oxidation number of fluorine is always -1. e. g. KF - (O. N. of K = +1, O. N. of F = -1) OF 2 - (O. N. of O = +2, O. N of F = -1)
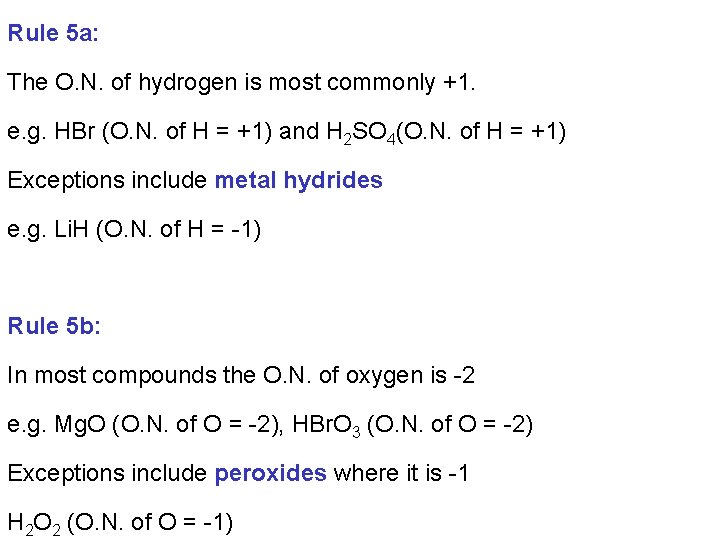
Rule 5 a: The O. N. of hydrogen is most commonly +1. e. g. HBr (O. N. of H = +1) and H 2 SO 4(O. N. of H = +1) Exceptions include metal hydrides e. g. Li. H (O. N. of H = -1) Rule 5 b: In most compounds the O. N. of oxygen is -2 e. g. Mg. O (O. N. of O = -2), HBr. O 3 (O. N. of O = -2) Exceptions include peroxides where it is -1 H 2 O 2 (O. N. of O = -1)

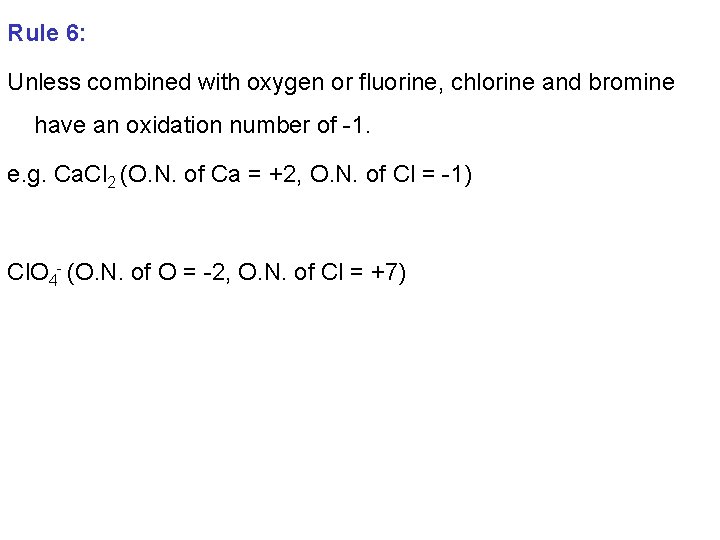
Rule 6: Unless combined with oxygen or fluorine, chlorine and bromine have an oxidation number of -1. e. g. Ca. Cl 2 (O. N. of Ca = +2, O. N. of Cl = -1) Cl. O 4 - (O. N. of O = -2, O. N. of Cl = +7)

Give the oxidation number for each element in the compounds below: H 2 O B 2 O 3 CO 2 Mn. O 2 Na 2 SO 4 Sn. O 2 H 2 O 2 Cl 2 Na. Cl. O N 2 Na. Cl. O 4 UO 2 PCl 5 H 2 S P 2 O 5 Na. H Ba. Cl 2 OF 2

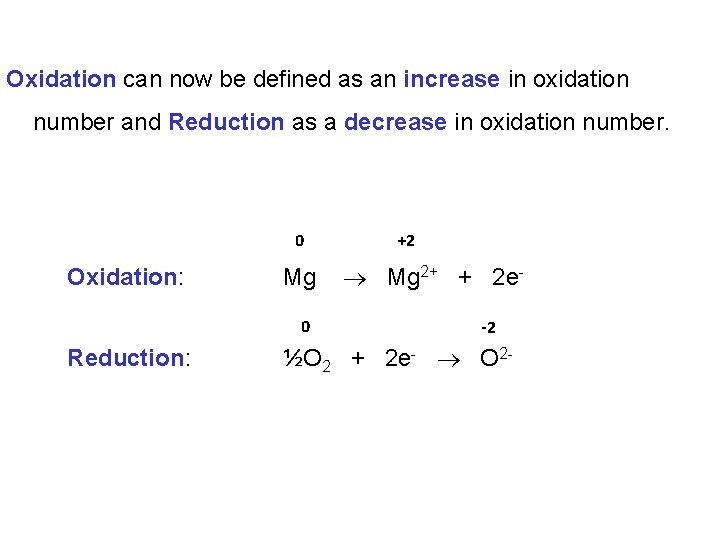
Oxidation can now be defined as an increase in oxidation number and Reduction as a decrease in oxidation number. 0 Oxidation: Mg 0 Reduction: +2 Mg 2+ + 2 e-2 ½O 2 + 2 e- O 2 -

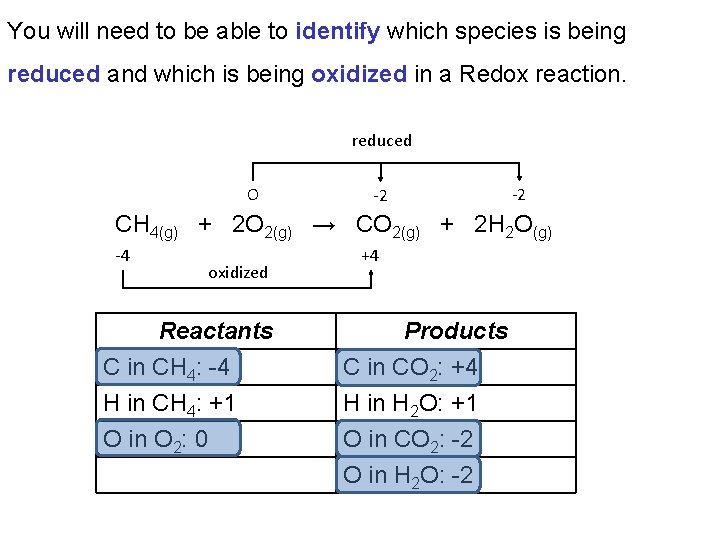
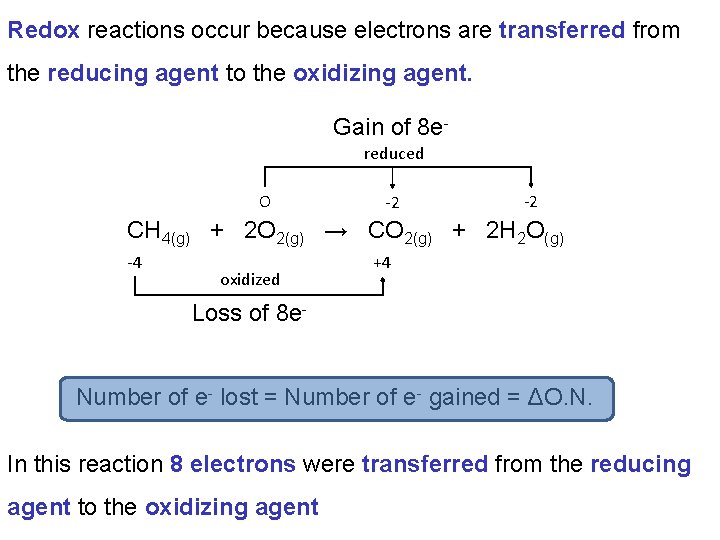
You will need to be able to identify which species is being reduced and which is being oxidized in a Redox reaction. reduced O -2 -2 CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(g) -4 oxidized Reactants C in CH 4: -4 H in CH 4: +1 O in O 2: 0 +4 Products C in CO 2: +4 H in H 2 O: +1 O in CO 2: -2 O in H 2 O: -2

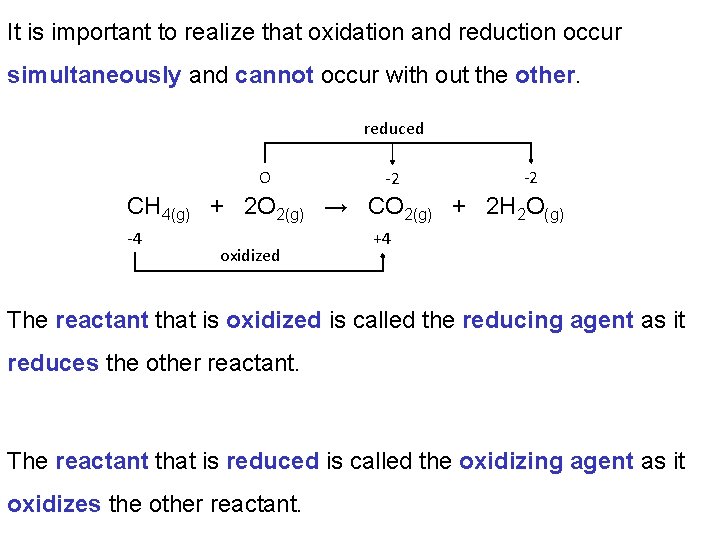
It is important to realize that oxidation and reduction occur simultaneously and cannot occur with out the other. reduced O -2 -2 CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(g) -4 oxidized +4 The reactant that is oxidized is called the reducing agent as it reduces the other reactant. The reactant that is reduced is called the oxidizing agent as it oxidizes the other reactant.

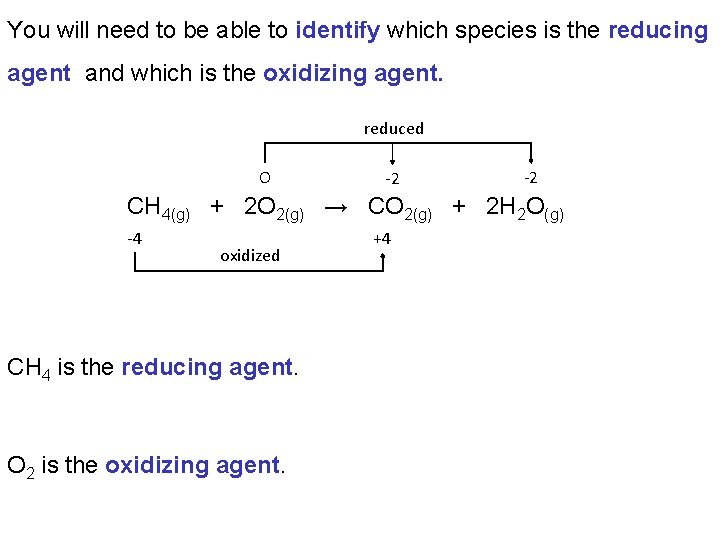
You will need to be able to identify which species is the reducing agent and which is the oxidizing agent. reduced O -2 -2 CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(g) -4 oxidized CH 4 is the reducing agent. O 2 is the oxidizing agent. +4

Redox reactions occur because electrons are transferred from the reducing agent to the oxidizing agent. Gain of 8 ereduced O -2 -2 CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(g) -4 oxidized +4 Loss of 8 e. Number of e- lost = Number of e- gained = ΔO. N. In this reaction 8 electrons were transferred from the reducing agent to the oxidizing agent

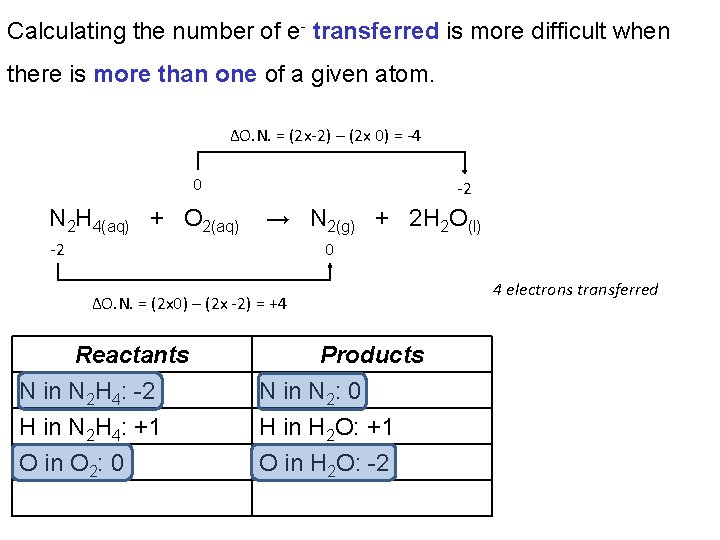
Calculating the number of e- transferred is more difficult when there is more than one of a given atom. ΔO. N. = (2 x-2) – (2 x 0) = -4 0 N 2 H 4(aq) + O 2(aq) -2 → N 2(g) + 2 H 2 O(l) -2 0 ΔO. N. = (2 x 0) – (2 x -2) = +4 Reactants N in N 2 H 4: -2 H in N 2 H 4: +1 O in O 2: 0 Products N in N 2: 0 H in H 2 O: +1 O in H 2 O: -2 4 electrons transferred

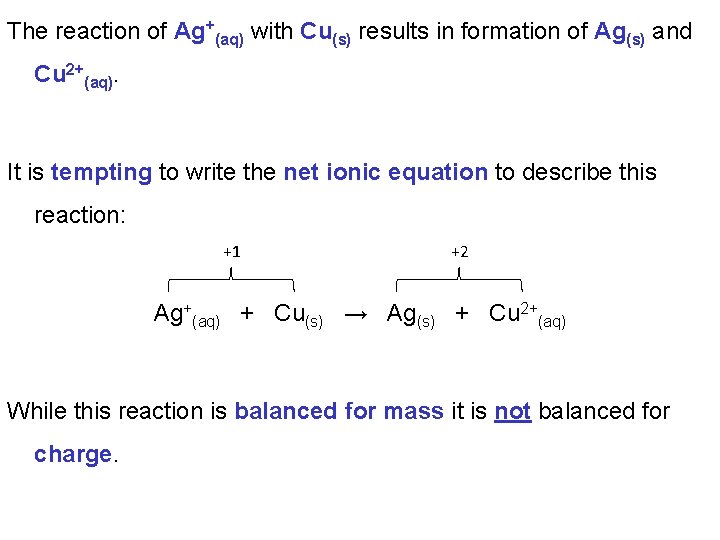
The reaction of Ag+(aq) with Cu(s) results in formation of Ag(s) and Cu 2+(aq). It is tempting to write the net ionic equation to describe this reaction: +1 +2 Ag+(aq) + Cu(s) → Ag(s) + Cu 2+(aq) While this reaction is balanced for mass it is not balanced for charge.

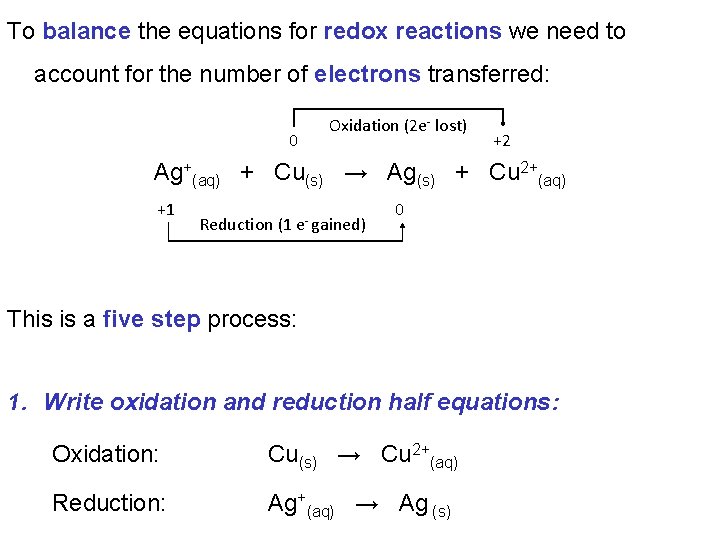
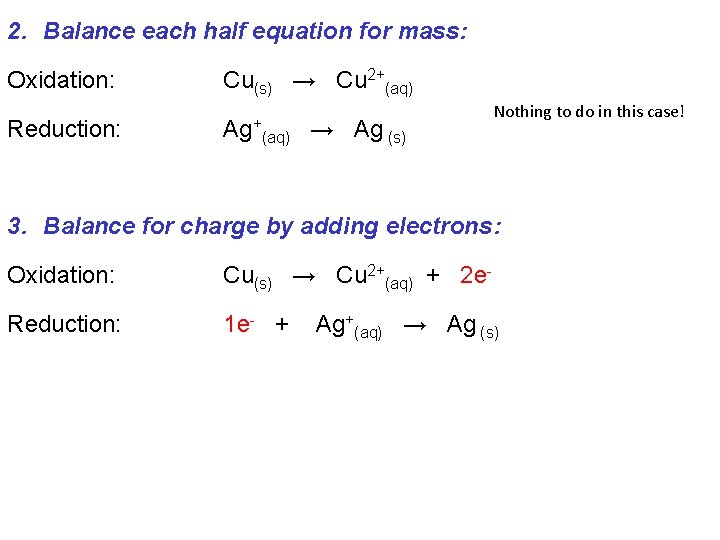
To balance the equations for redox reactions we need to account for the number of electrons transferred: 0 Oxidation (2 e- lost) +2 Ag+(aq) + Cu(s) → Ag(s) + Cu 2+(aq) +1 Reduction (1 e- gained) 0 This is a five step process: 1. Write oxidation and reduction half equations: Oxidation: Cu(s) → Cu 2+(aq) Reduction: Ag+(aq) → Ag (s)

2. Balance each half equation for mass: Oxidation: Cu(s) → Cu 2+(aq) Reduction: Ag+ (aq) → Ag (s) Nothing to do in this case! 3. Balance for charge by adding electrons: Oxidation: Cu(s) → Cu 2+(aq) + 2 e- Reduction: 1 e- + Ag+(aq) → Ag (s)

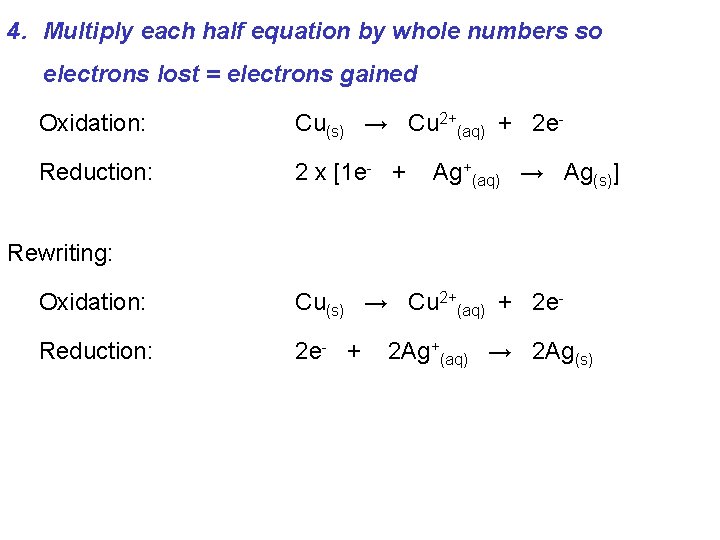
4. Multiply each half equation by whole numbers so electrons lost = electrons gained Oxidation: Cu(s) → Cu 2+(aq) + 2 e- Reduction: 2 x [1 e- + Ag+(aq) → Ag(s)] Rewriting: Oxidation: Cu(s) → Cu 2+(aq) + 2 e- Reduction: 2 e- + 2 Ag+(aq) → 2 Ag(s)

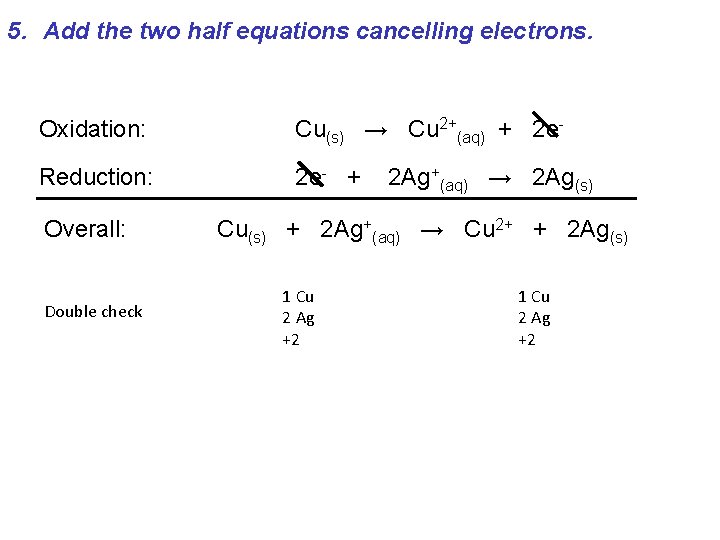
5. Add the two half equations cancelling electrons. Oxidation: Cu(s) → Cu 2+(aq) + 2 e- Reduction: 2 e- + Overall: Double check 2 Ag+(aq) → 2 Ag(s) Cu(s) + 2 Ag+(aq) → Cu 2+ + 2 Ag(s) 1 Cu 2 Ag +2

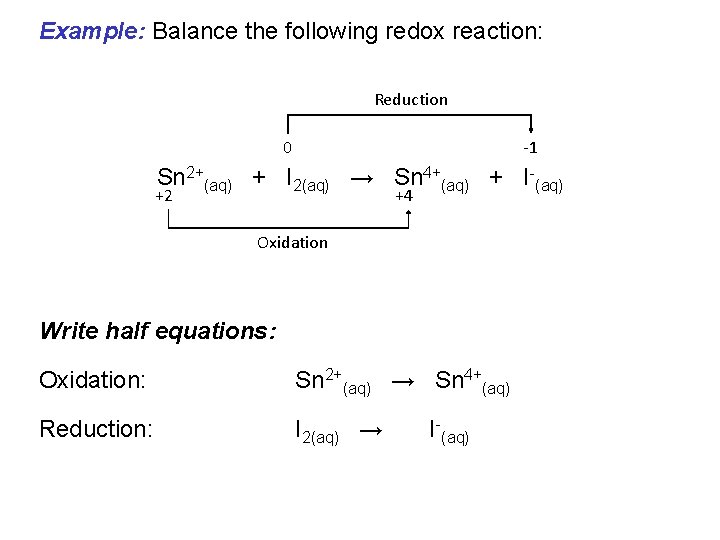
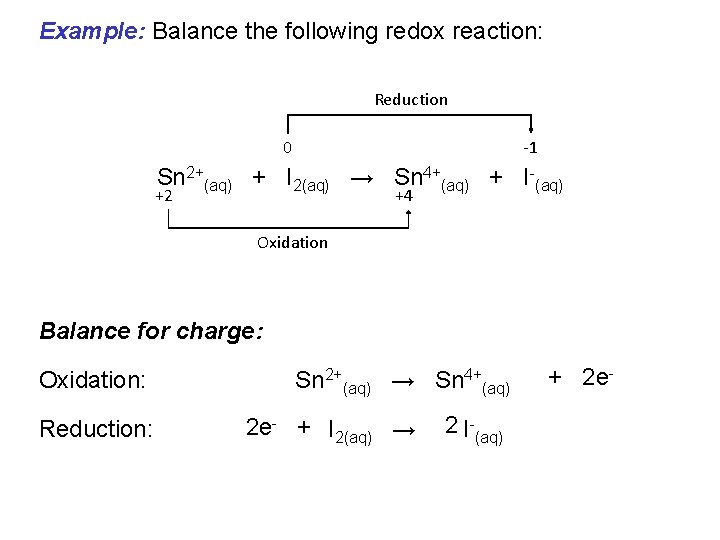
Example: Balance the following redox reaction: Reduction 0 -1 Sn 2+(aq) + I 2(aq) → Sn 4+(aq) + I-(aq) +2 +4 Oxidation Write half equations: Oxidation: Sn 2+(aq) → Sn 4+(aq) Reduction: I 2(aq) → I-(aq)

Example: Balance the following redox reaction: Reduction 0 -1 Sn 2+(aq) + I 2(aq) → Sn 4+(aq) + I-(aq) +2 +4 Oxidation Balance for mass: Oxidation: Sn 2+(aq) → Sn 4+(aq) Reduction: I 2(aq) → 2 I-(aq)

Example: Balance the following redox reaction: Reduction 0 -1 Sn 2+(aq) + I 2(aq) → Sn 4+(aq) + I-(aq) +2 +4 Oxidation Balance for charge: Oxidation: Reduction: Sn 2+(aq) → Sn 4+(aq) 2 e- + I 2(aq) → 2 I-(aq) + 2 e-

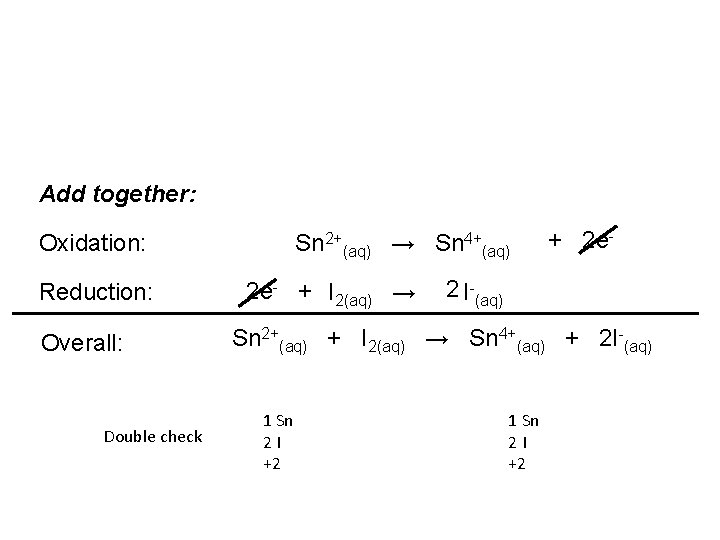
Add together: Oxidation: Reduction: Overall: Double check Sn 2+(aq) → Sn 4+(aq) 2 e- + I 2(aq) → + 2 e- 2 I-(aq) Sn 2+(aq) + I 2(aq) → Sn 4+(aq) + 2 I-(aq) 1 Sn 2 I +2

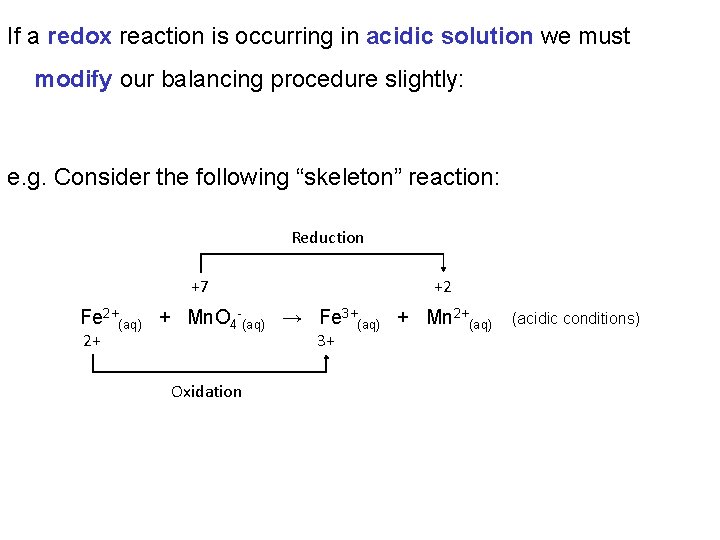
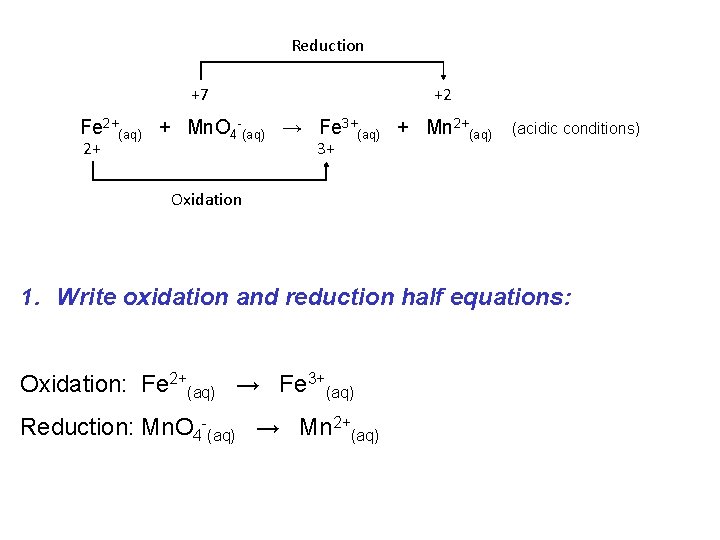
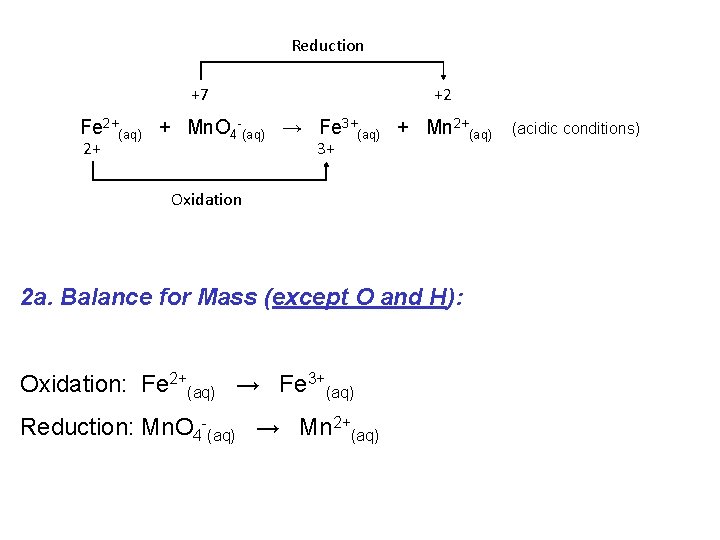
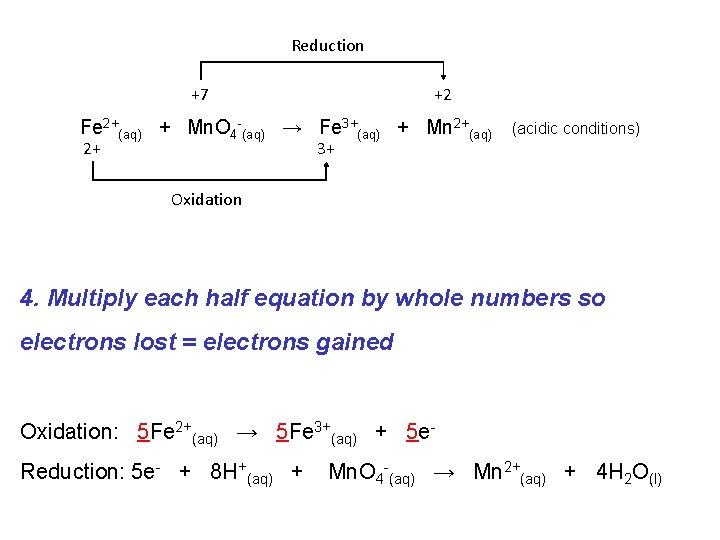
If a redox reaction is occurring in acidic solution we must modify our balancing procedure slightly: e. g. Consider the following “skeleton” reaction: Reduction +7 +2 Fe 2+(aq) + Mn. O 4 -(aq) → Fe 3+(aq) + Mn 2+(aq) 2+ 3+ Oxidation (acidic conditions)

Reduction +7 +2 Fe 2+(aq) + Mn. O 4 -(aq) → Fe 3+(aq) + Mn 2+(aq) 2+ 3+ (acidic conditions) Oxidation 1. Write oxidation and reduction half equations: Oxidation: Fe 2+(aq) → Fe 3+(aq) Reduction: Mn. O 4 -(aq) → Mn 2+(aq)

Reduction +7 +2 Fe 2+(aq) + Mn. O 4 -(aq) → Fe 3+(aq) + Mn 2+(aq) 2+ 3+ Oxidation 2 a. Balance for Mass (except O and H): Oxidation: Fe 2+(aq) → Fe 3+(aq) Reduction: Mn. O 4 -(aq) → Mn 2+(aq) (acidic conditions)

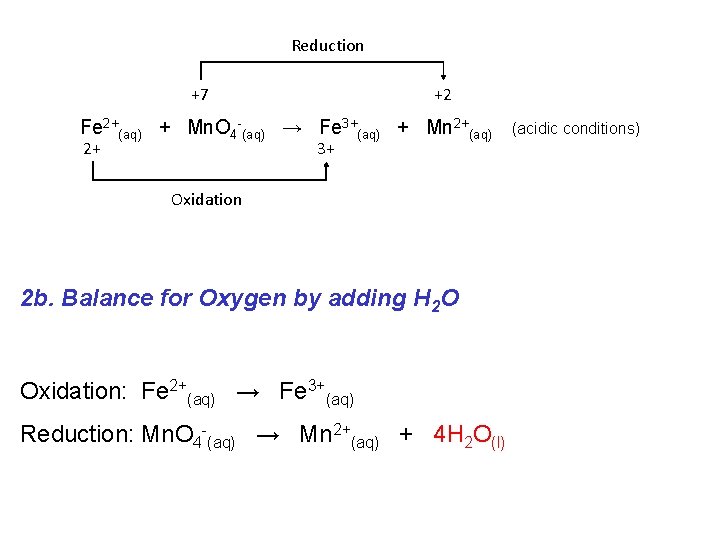
Reduction +7 +2 Fe 2+(aq) + Mn. O 4 -(aq) → Fe 3+(aq) + Mn 2+(aq) 2+ 3+ Oxidation 2 b. Balance for Oxygen by adding H 2 O Oxidation: Fe 2+(aq) → Fe 3+(aq) Reduction: Mn. O 4 -(aq) → Mn 2+(aq) + 4 H 2 O(l) (acidic conditions)

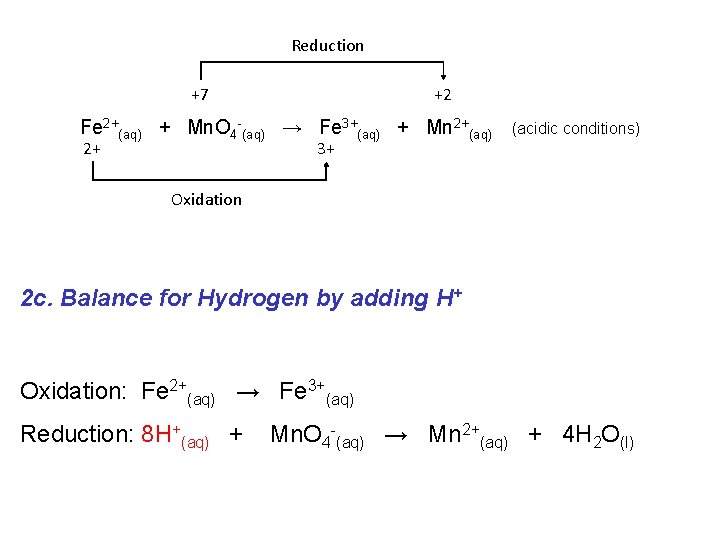
Reduction +7 +2 Fe 2+(aq) + Mn. O 4 -(aq) → Fe 3+(aq) + Mn 2+(aq) 2+ 3+ (acidic conditions) Oxidation 2 c. Balance for Hydrogen by adding H+ Oxidation: Fe 2+(aq) → Fe 3+(aq) Reduction: 8 H+(aq) + Mn. O 4 -(aq) → Mn 2+(aq) + 4 H 2 O(l)

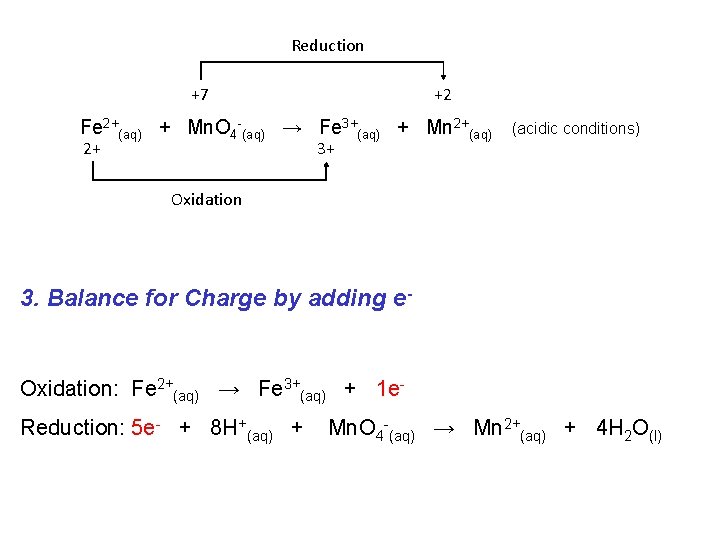
Reduction +7 +2 Fe 2+(aq) + Mn. O 4 -(aq) → Fe 3+(aq) + Mn 2+(aq) 2+ 3+ (acidic conditions) Oxidation 3. Balance for Charge by adding e- Oxidation: Fe 2+(aq) → Fe 3+(aq) + 1 e. Reduction: 5 e- + 8 H+(aq) + Mn. O 4 -(aq) → Mn 2+(aq) + 4 H 2 O(l)

Reduction +7 +2 Fe 2+(aq) + Mn. O 4 -(aq) → Fe 3+(aq) + Mn 2+(aq) 2+ 3+ (acidic conditions) Oxidation 4. Multiply each half equation by whole numbers so electrons lost = electrons gained Oxidation: 5 Fe 2+(aq) → 5 Fe 3+(aq) + 5 e. Reduction: 5 e- + 8 H+(aq) + Mn. O 4 -(aq) → Mn 2+(aq) + 4 H 2 O(l)

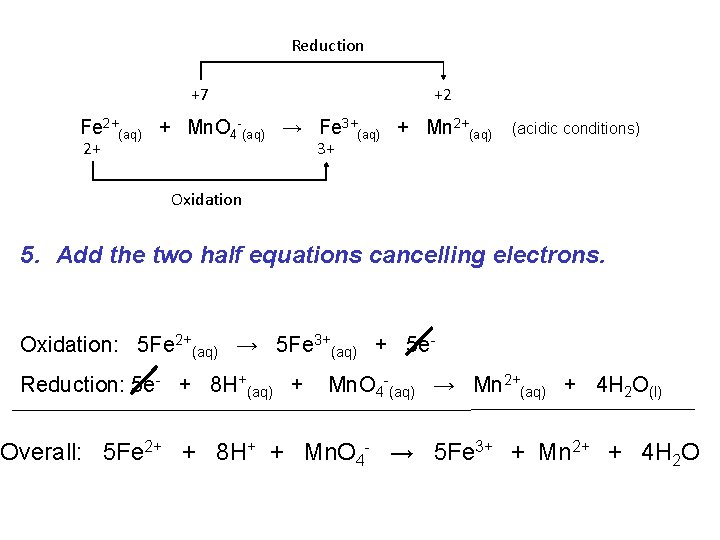
Reduction +7 +2 Fe 2+(aq) + Mn. O 4 -(aq) → Fe 3+(aq) + Mn 2+(aq) 2+ 3+ (acidic conditions) Oxidation 5. Add the two half equations cancelling electrons. Oxidation: 5 Fe 2+(aq) → 5 Fe 3+(aq) + 5 e. Reduction: 5 e- + 8 H+(aq) + Mn. O 4 -(aq) → Mn 2+(aq) + 4 H 2 O(l) Overall: 5 Fe 2+ + 8 H+ + Mn. O 4 - → 5 Fe 3+ + Mn 2+ + 4 H 2 O

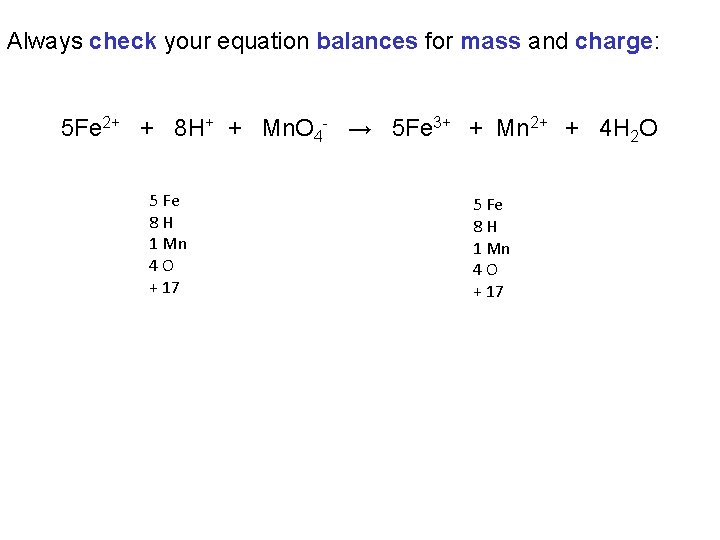
Always check your equation balances for mass and charge: 5 Fe 2+ + 8 H+ + Mn. O 4 - → 5 Fe 3+ + Mn 2+ + 4 H 2 O 5 Fe 8 H 1 Mn 4 O + 17

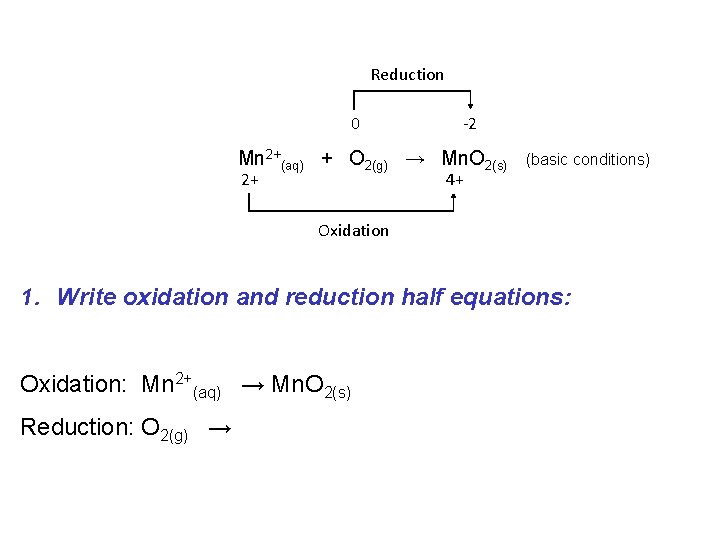
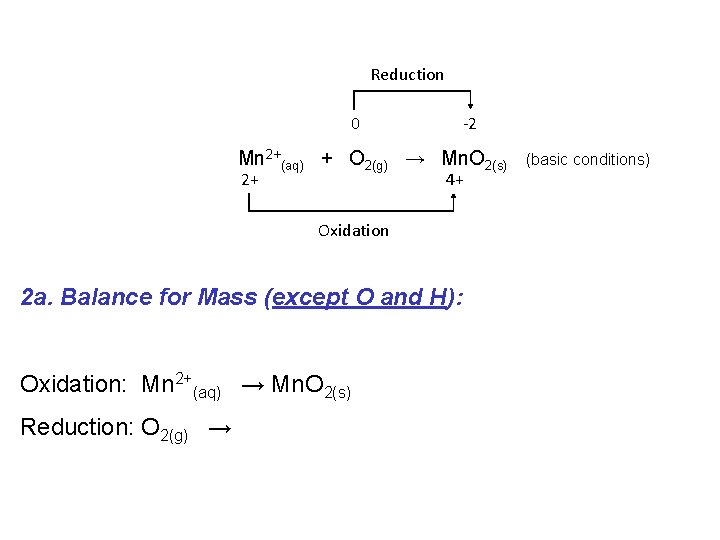
If a redox reaction is occurring in basic solution the procedure is similar as for acidic solution with one additional final step: e. g. Consider the following “skeleton” reaction: Reduction 0 -2 Mn 2+(aq) + O 2(g) → Mn. O 2(s) 2+ 4+ Oxidation (basic conditions)

Reduction 0 -2 Mn 2+(aq) + O 2(g) → Mn. O 2(s) 2+ 4+ (basic conditions) Oxidation 1. Write oxidation and reduction half equations: Oxidation: Mn 2+(aq) → Mn. O 2(s) Reduction: O 2(g) →

Reduction 0 -2 Mn 2+(aq) + O 2(g) → Mn. O 2(s) 2+ 4+ Oxidation 2 a. Balance for Mass (except O and H): Oxidation: Mn 2+(aq) → Mn. O 2(s) Reduction: O 2(g) → (basic conditions)

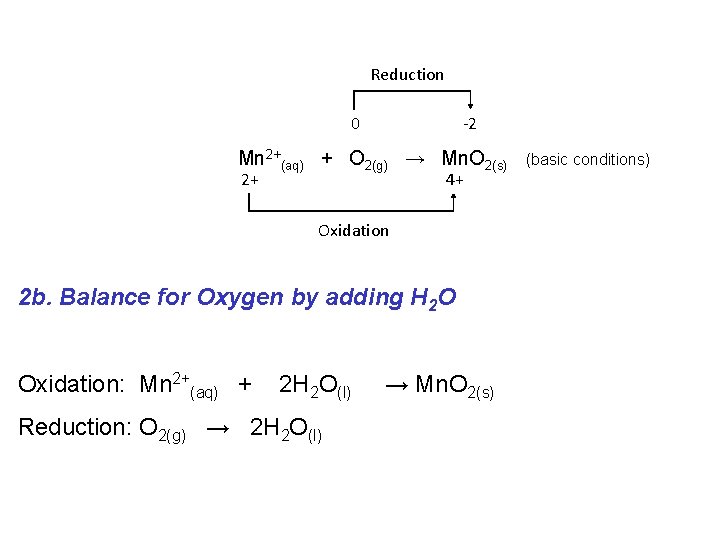
Reduction 0 -2 Mn 2+(aq) + O 2(g) → Mn. O 2(s) 2+ 4+ Oxidation 2 b. Balance for Oxygen by adding H 2 O Oxidation: Mn 2+(aq) + 2 H 2 O(l) Reduction: O 2(g) → 2 H 2 O(l) → Mn. O 2(s) (basic conditions)

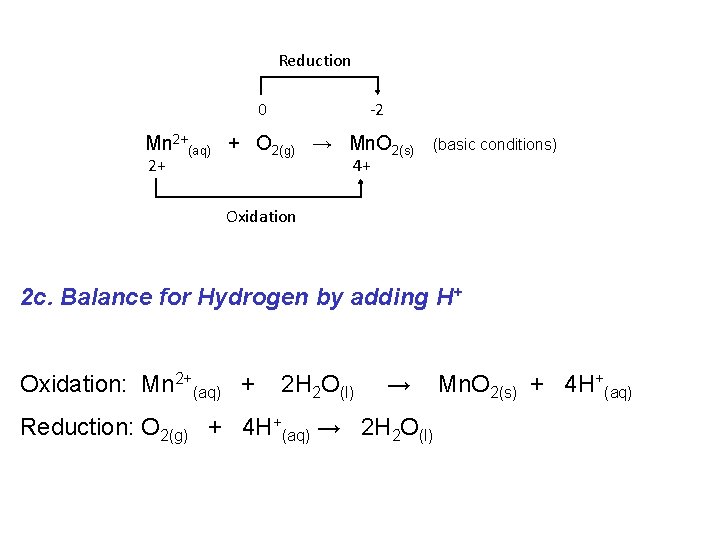
Reduction 0 -2 Mn 2+(aq) + O 2(g) → Mn. O 2(s) 2+ (basic conditions) 4+ Oxidation 2 c. Balance for Hydrogen by adding H+ Oxidation: Mn 2+(aq) + 2 H 2 O(l) → Reduction: O 2(g) + 4 H+(aq) → 2 H 2 O(l) Mn. O 2(s) + 4 H+(aq)

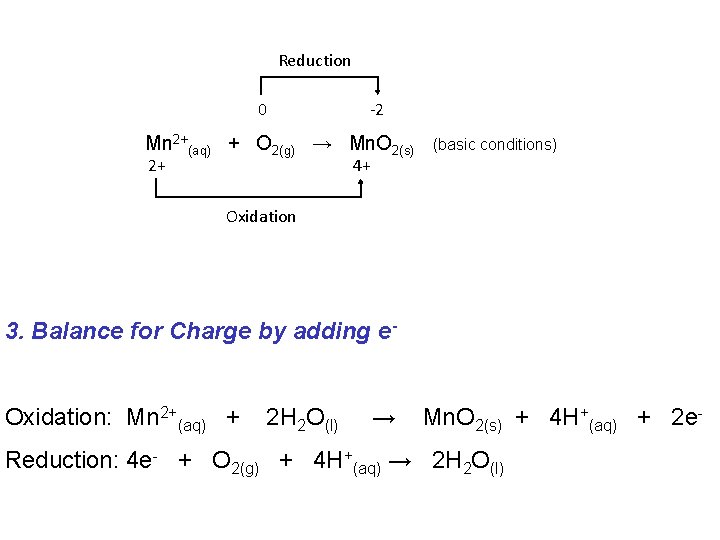
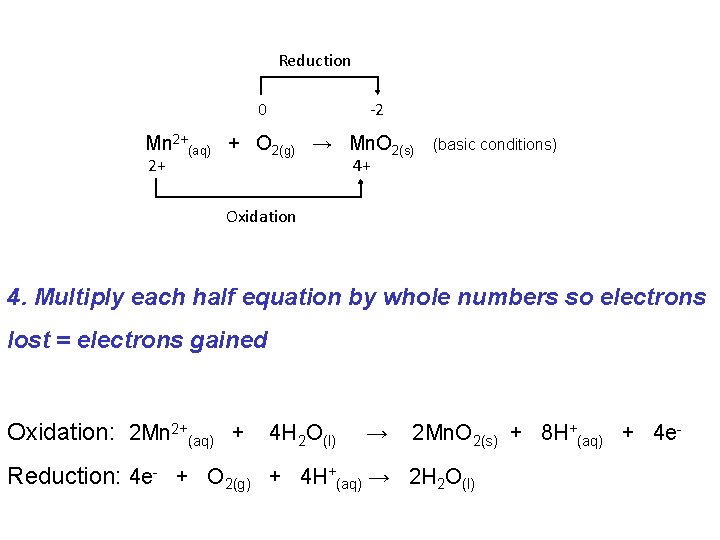
Reduction 0 -2 Mn 2+(aq) + O 2(g) → Mn. O 2(s) 2+ (basic conditions) 4+ Oxidation 3. Balance for Charge by adding e. Oxidation: Mn 2+(aq) + 2 H 2 O(l) → Mn. O 2(s) + 4 H+(aq) + 2 e- Reduction: 4 e- + O 2(g) + 4 H+(aq) → 2 H 2 O(l)

Reduction 0 -2 Mn 2+(aq) + O 2(g) → Mn. O 2(s) 2+ (basic conditions) 4+ Oxidation 4. Multiply each half equation by whole numbers so electrons lost = electrons gained Oxidation: 2 Mn 2+(aq) + 4 H 2 O(l) → 2 Mn. O 2(s) + 8 H+(aq) + 4 e- Reduction: 4 e- + O 2(g) + 4 H+(aq) → 2 H 2 O(l)

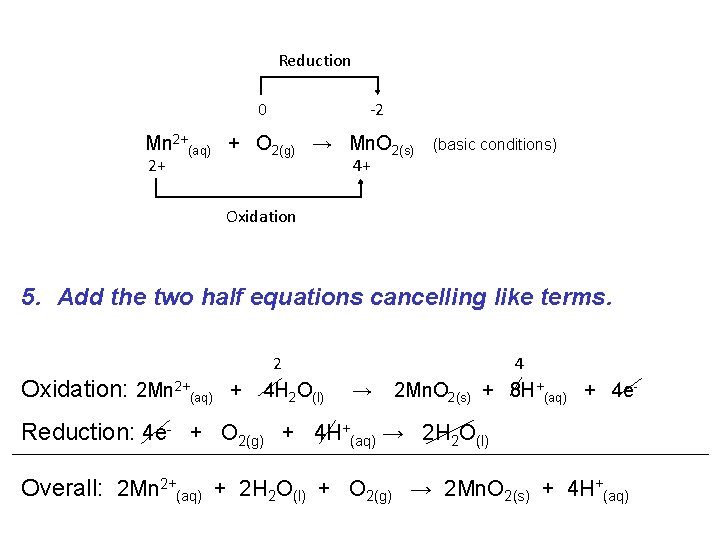
Reduction 0 -2 Mn 2+(aq) + O 2(g) → Mn. O 2(s) 2+ (basic conditions) 4+ Oxidation 5. Add the two half equations cancelling like terms. 2 Oxidation: 2 Mn 2+(aq) + 4 H 2 O(l) 4 → 2 Mn. O 2(s) + 8 H+(aq) + 4 e- Reduction: 4 e- + O 2(g) + 4 H+(aq) → 2 H 2 O(l) Overall: 2 Mn 2+(aq) + 2 H 2 O(l) + O 2(g) → 2 Mn. O 2(s) + 4 H+(aq)

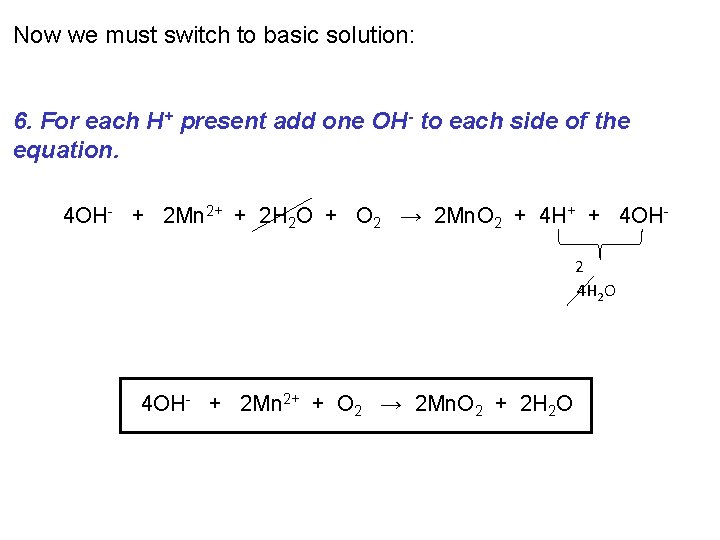
Now we must switch to basic solution: 6. For each H+ present add one OH- to each side of the equation. 4 OH- + 2 Mn 2+ + 2 H 2 O + O 2 → 2 Mn. O 2 + 4 H+ + 4 OH 2 4 H 2 O 4 OH- + 2 Mn 2+ + O 2 → 2 Mn. O 2 + 2 H 2 O

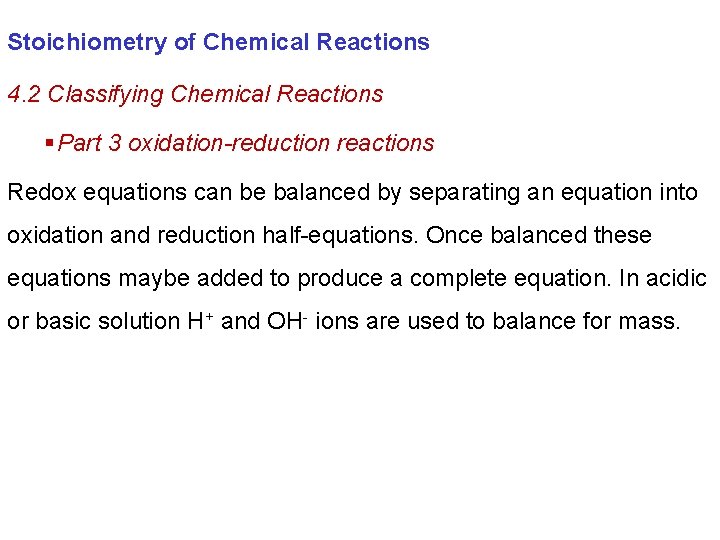
Stoichiometry of Chemical Reactions 4. 2 Classifying Chemical Reactions § Part 3 oxidation-reduction reactions Redox equations can be balanced by separating an equation into oxidation and reduction half-equations. Once balanced these equations maybe added to produce a complete equation. In acidic or basic solution H+ and OH- ions are used to balance for mass.
 General chemistry 1 stoichiometry
General chemistry 1 stoichiometry Chemical rxns/balancing equ./stoichiometry
Chemical rxns/balancing equ./stoichiometry Stoichiometry refers to
Stoichiometry refers to Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Chemical accounting stoichiometry
Chemical accounting stoichiometry Chemical accounting stoichiometry
Chemical accounting stoichiometry Stoichiometry map for chemical reactions
Stoichiometry map for chemical reactions Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Chapter 9 review stoichiometry section 1 answer key
Chapter 9 review stoichiometry section 1 answer key Ap chemistry stoichiometry
Ap chemistry stoichiometry Define reaction stoichiometry
Define reaction stoichiometry Chapter 11 stoichiometry test answer key
Chapter 11 stoichiometry test answer key Chapter 9 review stoichiometry answer key
Chapter 9 review stoichiometry answer key Ap chemistry stoichiometry
Ap chemistry stoichiometry Psalm 96 2-3
Psalm 96 2-3 Ds van wijk deventer
Ds van wijk deventer Mini vidas parts
Mini vidas parts Port 161
Port 161 Opwekking 585
Opwekking 585 Convenio 161 oit resumen
Convenio 161 oit resumen Cs161 ucr
Cs161 ucr Computer security 161 cryptocurrency lecture
Computer security 161 cryptocurrency lecture Jelena đorđevic 161
Jelena đorđevic 161 Inls 161
Inls 161 Computer science 161
Computer science 161 Astronomy 161
Astronomy 161 Mcp 161
Mcp 161 Glosf
Glosf Pa 161 program
Pa 161 program Ac 161
Ac 161 Error 161
Error 161 Drba-161
Drba-161 Csc 161 rochester
Csc 161 rochester Dbx 161
Dbx 161 Bvp 161
Bvp 161 Frank roorda
Frank roorda Jeroen sytsma
Jeroen sytsma Astronomy 161
Astronomy 161 160 161
160 161 Nabl assessor list
Nabl assessor list 180-161
180-161 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Empirical formula and molecular formula pogil
Empirical formula and molecular formula pogil Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Love formula in chemistry
Love formula in chemistry Are kc and kp equal
Are kc and kp equal What are the five chemical changes
What are the five chemical changes 5 general types of chemical reactions
5 general types of chemical reactions Combustion chemical reaction
Combustion chemical reaction Planos en cinematografia
Planos en cinematografia Where did general lee surrender to general grant?
Where did general lee surrender to general grant? Molecularity of reaction
Molecularity of reaction Pixl knowit gcse chemistry quantitative
Pixl knowit gcse chemistry quantitative Grade 11 chemistry unit 4
Grade 11 chemistry unit 4 Chapter 8 review chemical equations and reactions section 2
Chapter 8 review chemical equations and reactions section 2 A chemist shorthand way of representing chemical reaction
A chemist shorthand way of representing chemical reaction Chemistry chapter 9 chemical names and formulas
Chemistry chapter 9 chemical names and formulas Chemistry chapter 10 chemical quantities
Chemistry chapter 10 chemical quantities Ap chemistry equilibrium
Ap chemistry equilibrium Section 1 atoms elements and compounds
Section 1 atoms elements and compounds Chapter 6 section 1 atoms elements and compounds
Chapter 6 section 1 atoms elements and compounds General chemistry with qualitative analysis
General chemistry with qualitative analysis General chemistry thermochemistry
General chemistry thermochemistry General chemistry nomenclature
General chemistry nomenclature General chemistry 2
General chemistry 2 General chemistry petrucci
General chemistry petrucci General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry Exchange energy
Exchange energy General chemistry
General chemistry General chemistry
General chemistry Ib organic chemistry
Ib organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Gravimetric stoichiometry
Gravimetric stoichiometry Stoichiometry mole island diagram
Stoichiometry mole island diagram Gram to gram conversion
Gram to gram conversion Stoichiometry examples
Stoichiometry examples Molarity
Molarity Titration stoichiometry
Titration stoichiometry Stoichiometry game
Stoichiometry game Number of moles formula
Number of moles formula Gas stoichiometry worksheet
Gas stoichiometry worksheet Stoichiometry deals with..,
Stoichiometry deals with.., Stoichiometry is the study of
Stoichiometry is the study of What is composition stoichiometry
What is composition stoichiometry What stoichiometry
What stoichiometry Mol island
Mol island Stoichiometry
Stoichiometry Stoichiometry examples
Stoichiometry examples Stoichiometry is defined as the quantitative study of
Stoichiometry is defined as the quantitative study of Introduction to stoichiometry
Introduction to stoichiometry Stoicheion
Stoicheion Stoichiometry in greek
Stoichiometry in greek Stoichiometry cookie lab
Stoichiometry cookie lab Stoichiometry equation
Stoichiometry equation Cookie stoichiometry
Cookie stoichiometry Stoichiometry
Stoichiometry