Chapter 9 Stoichiometry Test REVIEW SHEET 1 A





- Slides: 11

Chapter 9 Stoichiometry Test REVIEW SHEET

1. A balanced chemical equation allows one to determine the ________ between all compounds in the equation. 1. MOLAR RATIOS 2. The coefficients in a chemical equation represent the ________ of reactants and products. 2. RELATIVE NUMBER OF MOLES 3. Actual yield of a product in a reaction must be determined by_____________. 3. EXPERIMENTS 4. Knowing the mole ratio of a reactant and product in a chemical reaction would allow you to determine the _____ and ______ of a product. 4. MOLES AND MASS

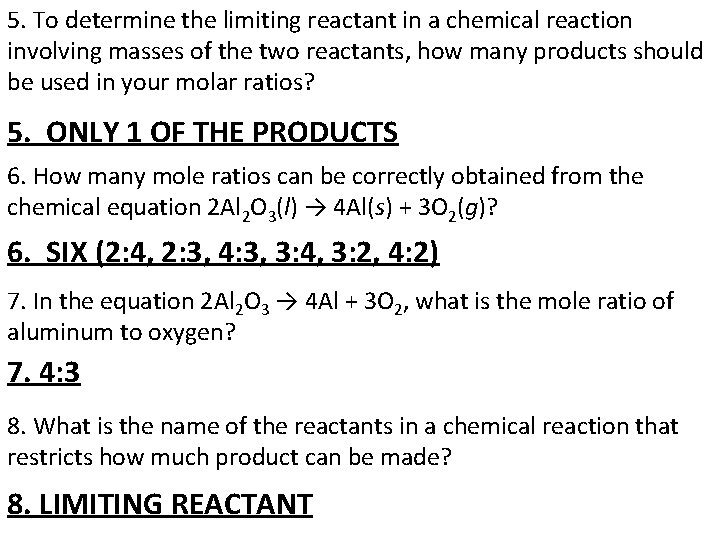
5. To determine the limiting reactant in a chemical reaction involving masses of the two reactants, how many products should be used in your molar ratios? 5. ONLY 1 OF THE PRODUCTS 6. How many mole ratios can be correctly obtained from the chemical equation 2 Al 2 O 3(l) → 4 Al(s) + 3 O 2(g)? 6. SIX (2: 4, 2: 3, 4: 3, 3: 4, 3: 2, 4: 2) 7. In the equation 2 Al 2 O 3 → 4 Al + 3 O 2, what is the mole ratio of aluminum to oxygen? 4: 3 7. 8. What is the name of the reactants in a chemical reaction that restricts how much product can be made? 8. LIMITING REACTANT

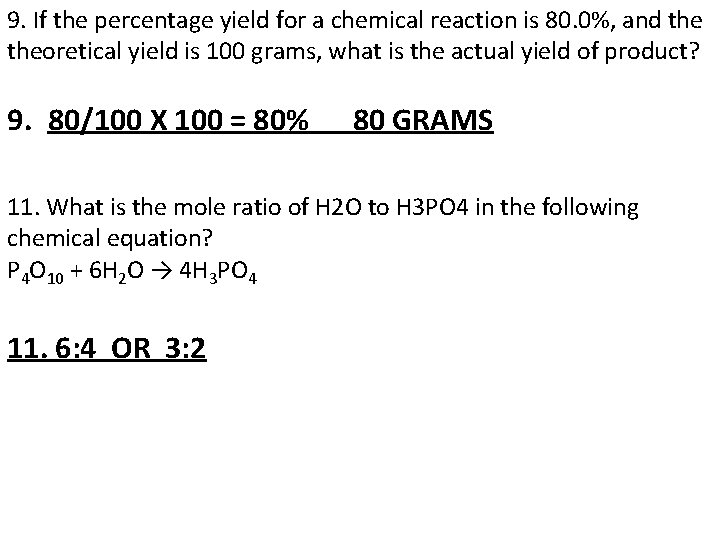
9. If the percentage yield for a chemical reaction is 80. 0%, and theoretical yield is 100 grams, what is the actual yield of product? 9. 80/100 X 100 = 80% 80 GRAMS 11. What is the mole ratio of H 2 O to H 3 PO 4 in the following chemical equation? P 4 O 10 + 6 H 2 O → 4 H 3 PO 4 11. 6: 4 OR 3: 2

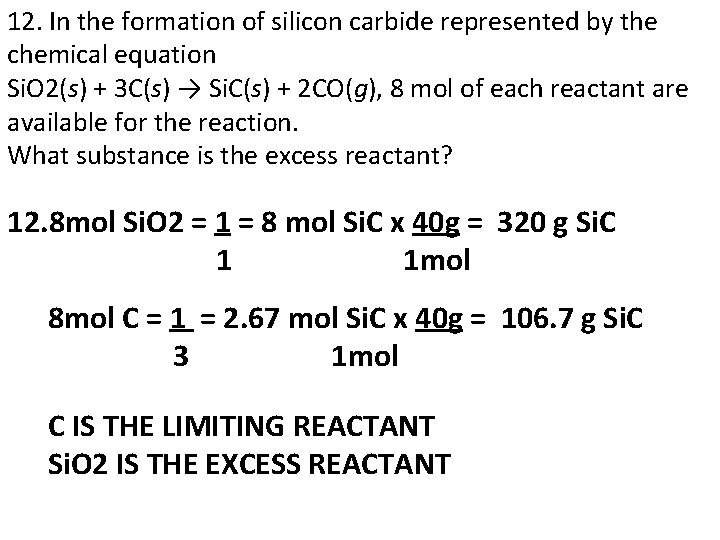
12. In the formation of silicon carbide represented by the chemical equation Si. O 2(s) + 3 C(s) → Si. C(s) + 2 CO(g), 8 mol of each reactant are available for the reaction. What substance is the excess reactant? 12. 8 mol Si. O 2 = 1 = 8 mol Si. C x 40 g = 320 g Si. C 1 1 mol 8 mol C = 1 = 2. 67 mol Si. C x 40 g = 106. 7 g Si. C 3 1 mol C IS THE LIMITING REACTANT Si. O 2 IS THE EXCESS REACTANT

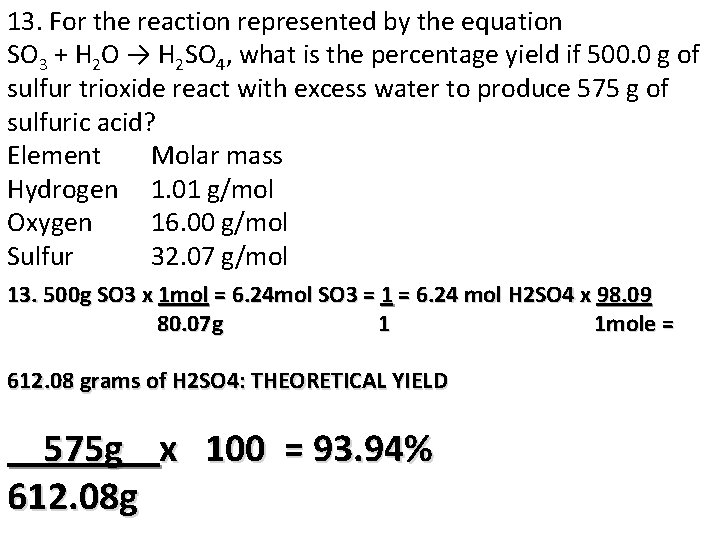
13. For the reaction represented by the equation SO 3 + H 2 O → H 2 SO 4, what is the percentage yield if 500. 0 g of sulfur trioxide react with excess water to produce 575 g of sulfuric acid? Element Molar mass Hydrogen 1. 01 g/mol Oxygen 16. 00 g/mol Sulfur 32. 07 g/mol 13. 500 g SO 3 x 1 mol = 6. 24 mol SO 3 = 1 = 6. 24 mol H 2 SO 4 x 98. 09 80. 07 g 1 1 mole = 612. 08 grams of H 2 SO 4: THEORETICAL YIELD 575 g x 100 = 93. 94% 612. 08 g

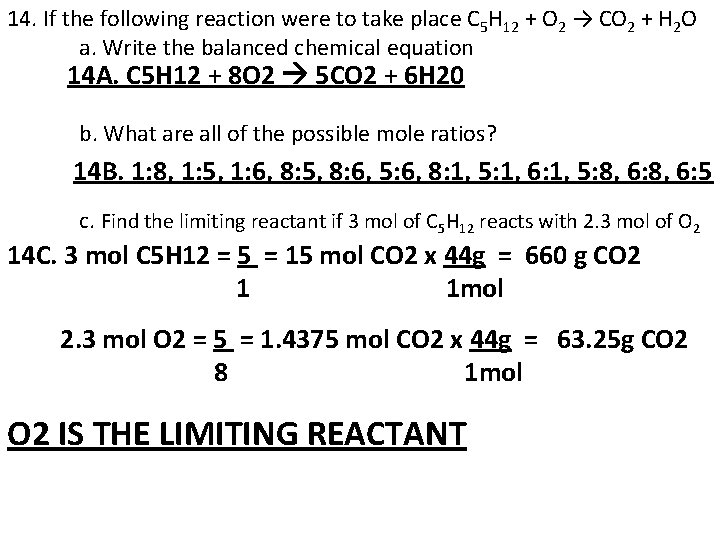
14. If the following reaction were to take place C 5 H 12 + O 2 → CO 2 + H 2 O a. Write the balanced chemical equation 14 A. C 5 H 12 + 8 O 2 5 CO 2 + 6 H 20 b. What are all of the possible mole ratios? 14 B. 1: 8, 1: 5, 1: 6, 8: 5, 8: 6, 5: 6, 8: 1, 5: 1, 6: 1, 5: 8, 6: 5 c. Find the limiting reactant if 3 mol of C 5 H 12 reacts with 2. 3 mol of O 2 14 C. 3 mol C 5 H 12 = 5 = 15 mol CO 2 x 44 g = 660 g CO 2 1 1 mol 2. 3 mol O 2 = 5 = 1. 4375 mol CO 2 x 44 g = 63. 25 g CO 2 8 1 mol O 2 IS THE LIMITING REACTANT

14. d. How much excess reactant would there be? 14 D. 2. 3 mol O 2 = 1 =. 2875 mol C 5 H 12 ACTUALLY USED UP 8 3 mol C 5 H 12 -. 2875 mol C 5 H 12 = 2. 7125 excess 14. e. How many grams of water would you expect to form? 14 E. 2. 3 mol O 2 = 6 = 1. 725 mol H 2 O x 18 g = 31. 05 g H 2 O 8 1 mol

15. If the following reaction were to take place Al + Fe 3 N 2 → Al. N + Fe a. Write the balanced chemical equation 15 A. 2 Al + Fe 3 N 2 2 Al. N + 3 Fe b. What are all of the possible mole ratios? 15 B. 2: 1, 15 C. 2: 2, 2: 3, 1: 2, 1: 3, 2: 3, 1: 2, 2: 2, 3: 2, 2: 1, 3: 2 c. Find the limiting reactant if 1. 7 mol of Al reacts with. 6 mol of Fe 3 N 2 1. 7 mol Al = 3 = 2. 55 mol Fe x 54 g = 137. 7 g Fe 2 1 mol . 6 mol Fe 3 N 2 = 3 = 1. 8 mol Fe x 1 54 g = 97. 2 g Fe 1 mol Fe 3 N 2 IS THE LIMITING REACTANT

15 d. How much excess reactant would there be? . 6 mol Fe 3 N 2 = 1. 2 mol Al ACTUALLY USED UP 1 1. 7 mol – 1. 2 mol =. 5 mol excess Al 15 e. How many grams of iron would you expect to form? . 6 mol Fe 3 N 2 = 3 = 1. 8 mol Fe x 54 g = 97. 2 g Fe 1 1 mol

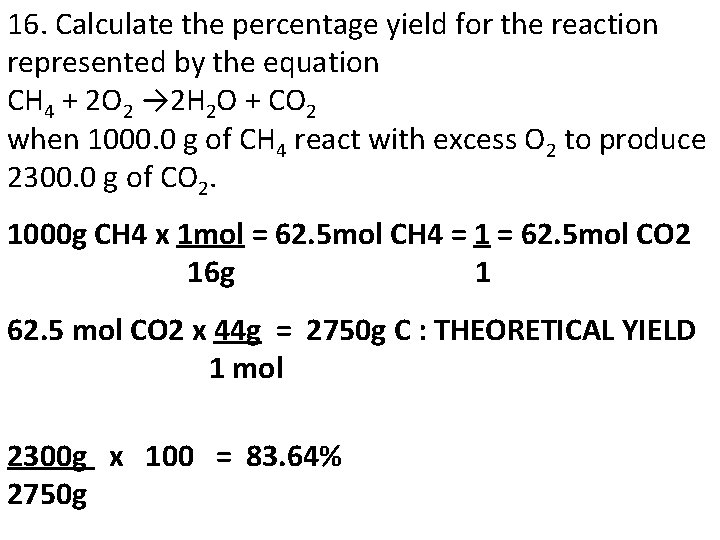
16. Calculate the percentage yield for the reaction represented by the equation CH 4 + 2 O 2 → 2 H 2 O + CO 2 when 1000. 0 g of CH 4 react with excess O 2 to produce 2300. 0 g of CO 2. 1000 g CH 4 x 1 mol = 62. 5 mol CH 4 = 1 = 62. 5 mol CO 2 16 g 1 62. 5 mol CO 2 x 44 g = 2750 g C : THEORETICAL YIELD 1 mol 2300 g x 100 = 83. 64% 2750 g