AP Chemistry Stoichiometry In thermite reaction a ture


- Slides: 14

AP Chemistry Stoichiometry In thermite reaction, a ture of powdered aluminum and powdered iron(III) oxide to yield iron and aluminum xide. The reaction burns hot ough to be useful in underwater welding. 2 Al + Fe 2 O 3 2 Fe + Al 2 O 3 + ener

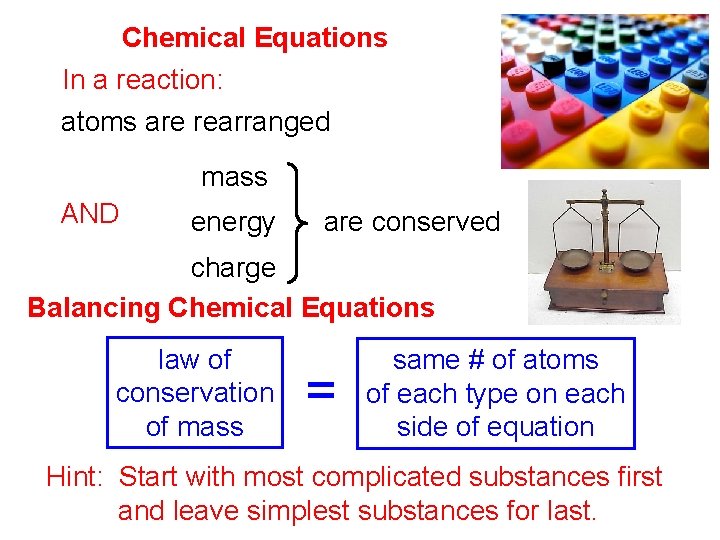
Chemical Equations In a reaction: atoms are rearranged mass AND energy are conserved charge Balancing Chemical Equations law of conservation of mass = same # of atoms of each type on each side of equation Hint: Start with most complicated substances first and leave simplest substances for last.

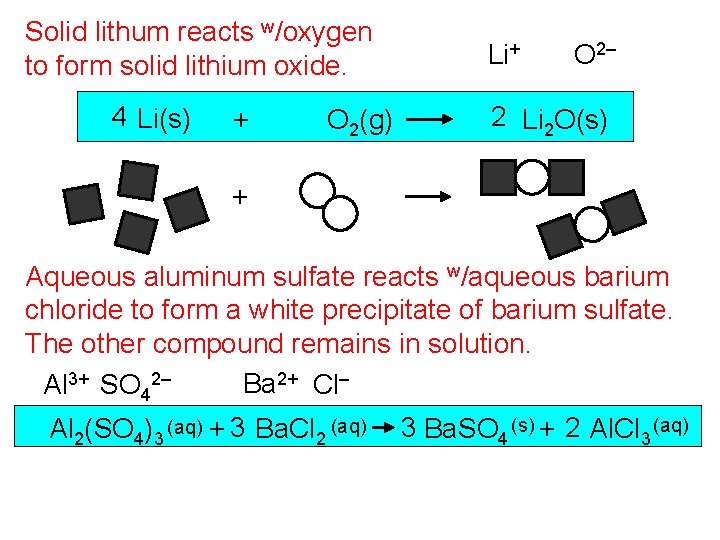
Solid lithum reacts w/oxygen to form solid lithium oxide. 4 Li(s) + O 2(g) Li+ O 2– 2 Li 2 O(s) + Aqueous aluminum sulfate reacts w/aqueous barium chloride to form a white precipitate of barium sulfate. The other compound remains in solution. Ba 2+ Cl– Al 3+ SO 42– Al 2(SO 4)3 (aq) + 3 Ba. Cl 2 (aq) 3 Ba. SO 4 (s) + 2 Al. Cl 3 (aq)

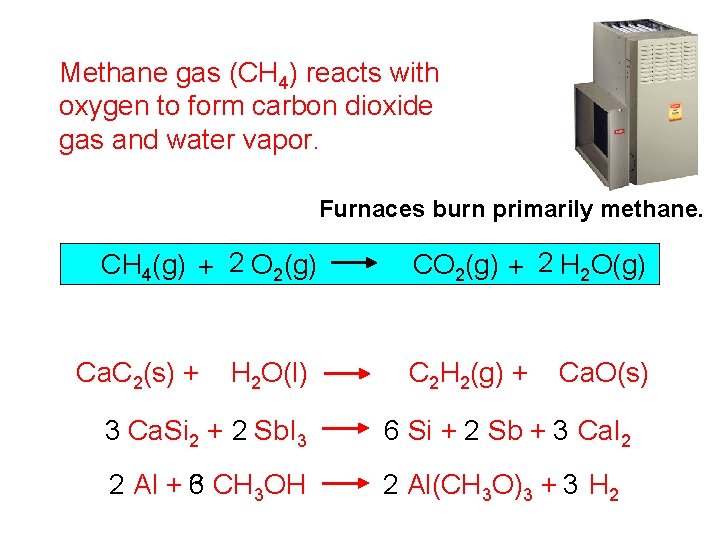
Methane gas (CH 4) reacts with oxygen to form carbon dioxide gas and water vapor. Furnaces burn primarily methane. CH 4(g) + 2 O 2(g) Ca. C 2(s) + H 2 O(l) CO 2(g) + 2 H 2 O(g) C 2 H 2(g) + Ca. O(s) 3 Ca. Si 2 + 2 Sb. I 3 6 Si + 2 Sb + 3 Ca. I 2 2 Al + 3 6 CH 3 OH 2 Al(CH 3 O)3 + 3 H 2

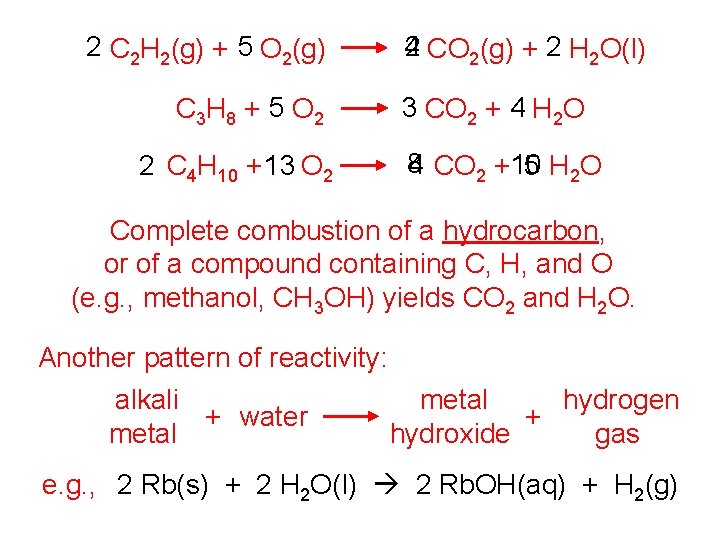
2 C 2 H 2(g) + 5 O 2(g) C 3 H 8 + 5 O 2 2 C 4 H 10 + 13 O 2 2 CO 2(g) + 2 H 2 O(l) 4 3 CO 2 + 4 H 2 O 8 4 CO 2 +10 5 H 2 O Complete combustion of a hydrocarbon, or of a compound containing C, H, and O (e. g. , methanol, CH 3 OH) yields CO 2 and H 2 O. Another pattern of reactivity: alkali metal hydrogen + water + metal hydroxide gas e. g. , 2 Rb(s) + 2 H 2 O(l) 2 Rb. OH(aq) + H 2(g)

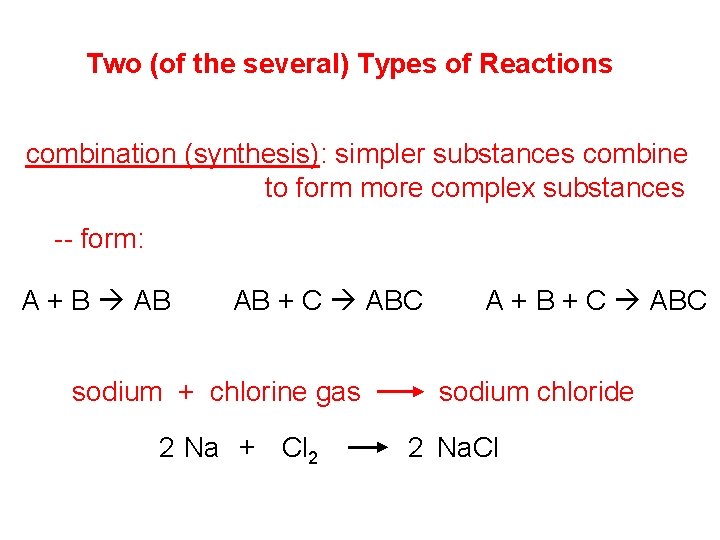
Two (of the several) Types of Reactions combination (synthesis): simpler substances combine to form more complex substances -- form: A + B AB AB + C ABC sodium + chlorine gas 2 Na + Cl 2 A + B + C ABC sodium chloride 2 Na. Cl

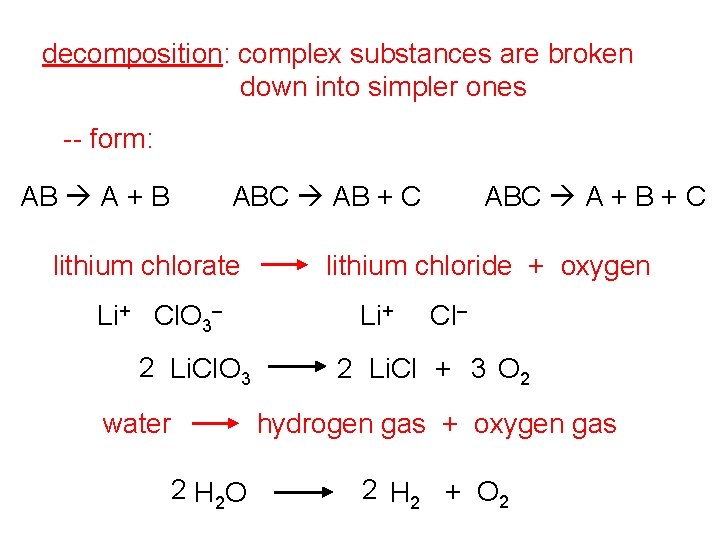
decomposition: complex substances are broken down into simpler ones -- form: AB A + B ABC AB + C lithium chlorate Li+ Cl. O 3– 2 Li. Cl. O 3 water ABC A + B + C lithium chloride + oxygen Li+ Cl– 2 Li. Cl + 3 O 2 hydrogen gas + oxygen gas 2 H 2 O 2 H 2 + O 2

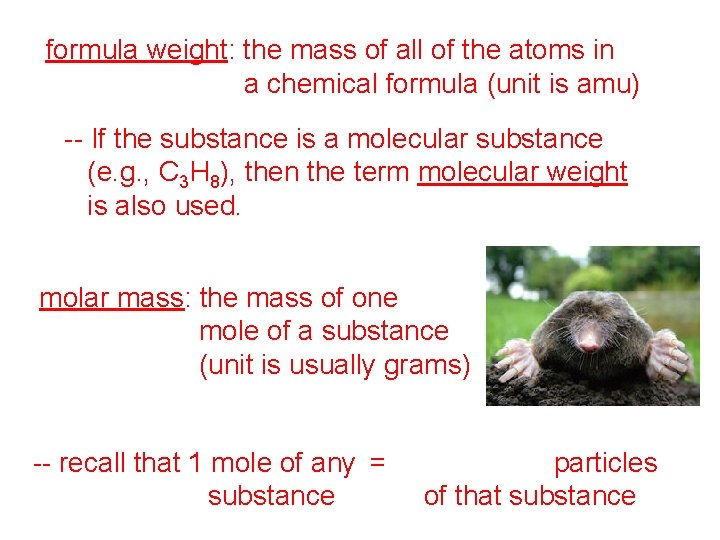
formula weight: the mass of all of the atoms in a chemical formula (unit is amu) -- If the substance is a molecular substance (e. g. , C 3 H 8), then the term molecular weight is also used. molar mass: the mass of one mole of a substance (unit is usually grams) -- recall that 1 mole of any = 6. 02 x 1023 particles substance of that substance

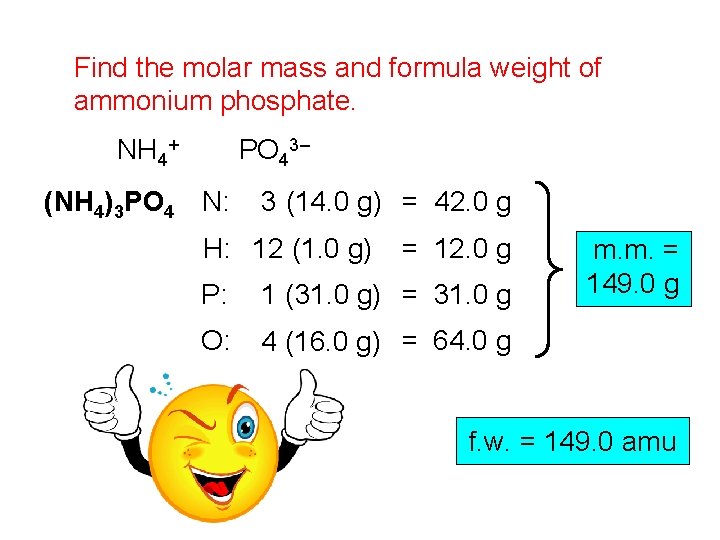
Find the molar mass and formula weight of ammonium phosphate. NH 4+ PO 43– (NH 4)3 PO 4 N: 3 (14. 0 g) = 42. 0 g H: 12 (1. 0 g) = 12. 0 g P: 1 (31. 0 g) = 31. 0 g O: 4 (16. 0 g) = 64. 0 g m. m. = 149. 0 g f. w. = 149. 0 amu

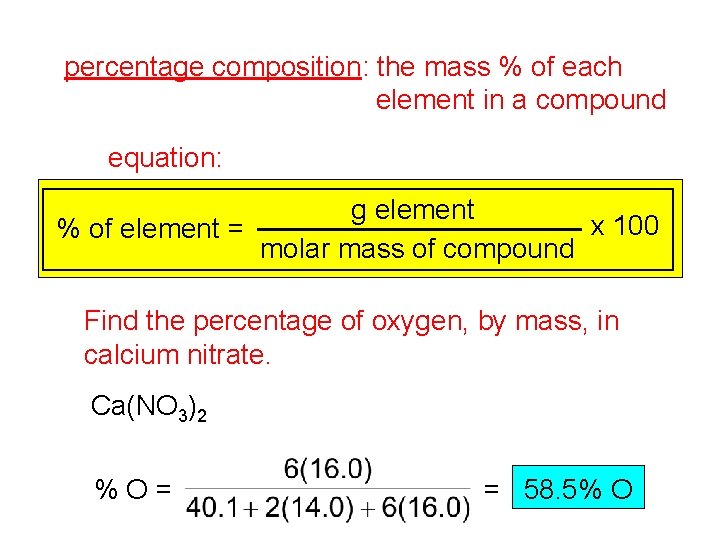
percentage composition: the mass % of each element in a compound equation: g element x 100 % of element = molar mass of compound Find the percentage of oxygen, by mass, in calcium nitrate. Ca(NO 3)2 %O= = 58. 5% O

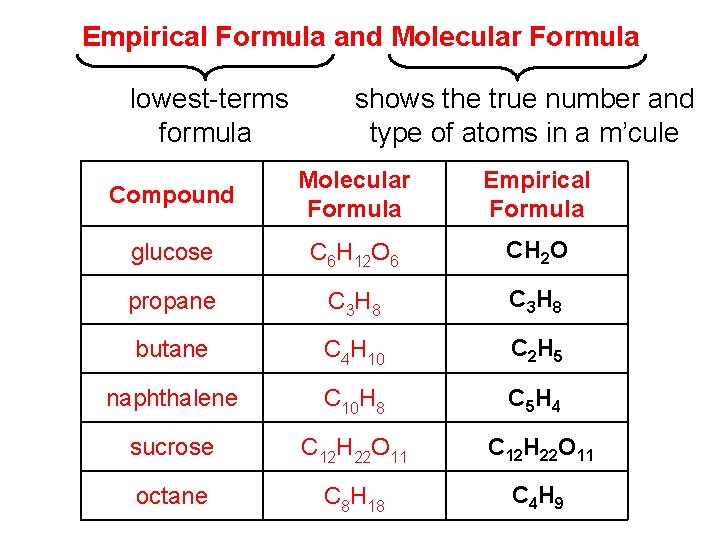
Empirical Formula and Molecular Formula lowest-terms formula shows the true number and type of atoms in a m’cule Compound Molecular Formula Empirical Formula glucose C 6 H 12 O 6 CH 2 O propane C 3 H 8 butane C 4 H 10 C 2 H 5 naphthalene C 10 H 8 C 5 H 4 sucrose C 12 H 22 O 11 octane C 8 H 18 C 4 H 9

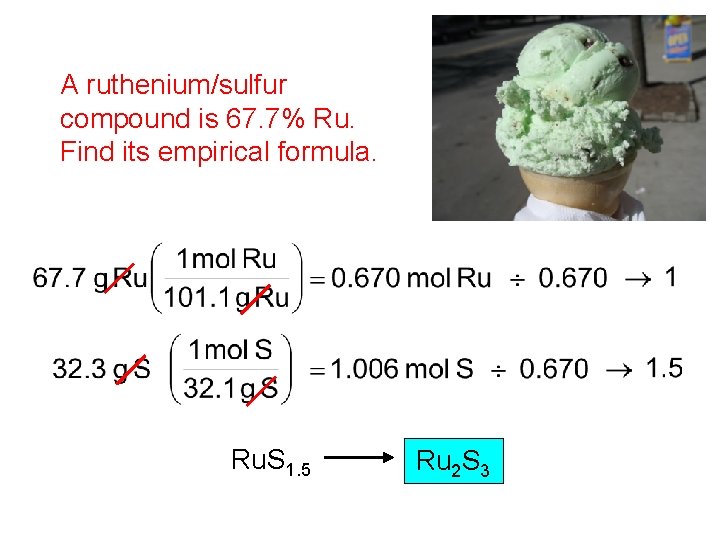
Finding an Empirical Formula from Experimental Data 1. Find # of g of each element. 2. Convert each g to mol. 3. Divide each “# of mol” by the smallest “# of mol. ” 4. Use ratio to find formula. “What’s your flavor of ice cream? ”

A ruthenium/sulfur compound is 67. 7% Ru. Find its empirical formula. Ru. S 1. 5 Ru 2 S 3

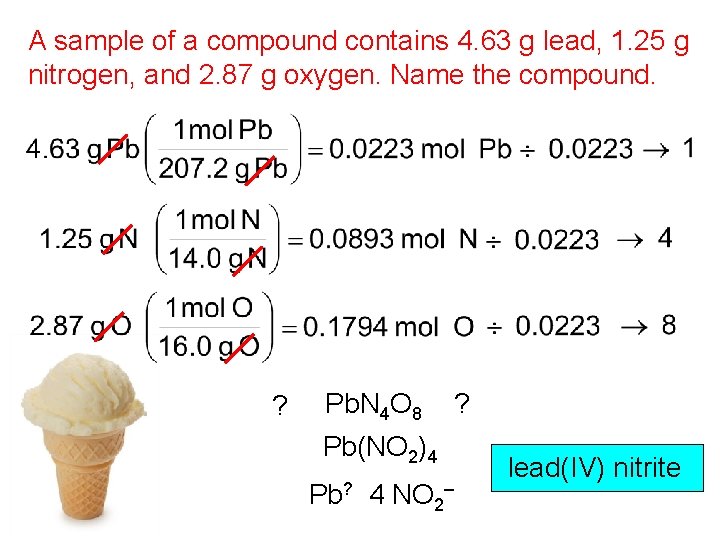
A sample of a compound contains 4. 63 g lead, 1. 25 g nitrogen, and 2. 87 g oxygen. Name the compound. ? Pb. N 4 O 8 ? Pb(NO 2)4 Pb? 4 NO 2– lead(IV) nitrite
 Göreli tür nedir
Göreli tür nedir Ap chemistry stoichiometry
Ap chemistry stoichiometry Thermite pot
Thermite pot Jembatan mol
Jembatan mol Türe özgü hazırbulunuşluk
Türe özgü hazırbulunuşluk Ture words
Ture words Meta yönlendirici gruplar
Meta yönlendirici gruplar 22620 in word
22620 in word Algısal ayırt edilebilirlik
Algısal ayırt edilebilirlik Suffixe ure
Suffixe ure Ture words
Ture words Define reaction stoichiometry
Define reaction stoichiometry Single replacement
Single replacement Reaction rate and stoichiometry
Reaction rate and stoichiometry In a chemical reaction, stoichiometry refers to
In a chemical reaction, stoichiometry refers to