Stoichiometry Chemistry II Chapter 9 Reaction Stoichiometry l









- Slides: 20

Stoichiometry Chemistry II Chapter 9

Reaction Stoichiometry l Based on conservation of matter – l Balanced equation If the quantity of one substance is known, others can be found – Mole ratios

5 Step Process 1. 2. 3. 4. 5. Construct a balanced equation. Identify known(s) and unknown(s). Convert known(s) to moles (if necessary). Find moles of unknown(s). Convert unknown(s) into required units (if necessary).

Example Problem How many grams of nitrogen will react with 112 L of hydrogen to form ammonia (NH 3)?

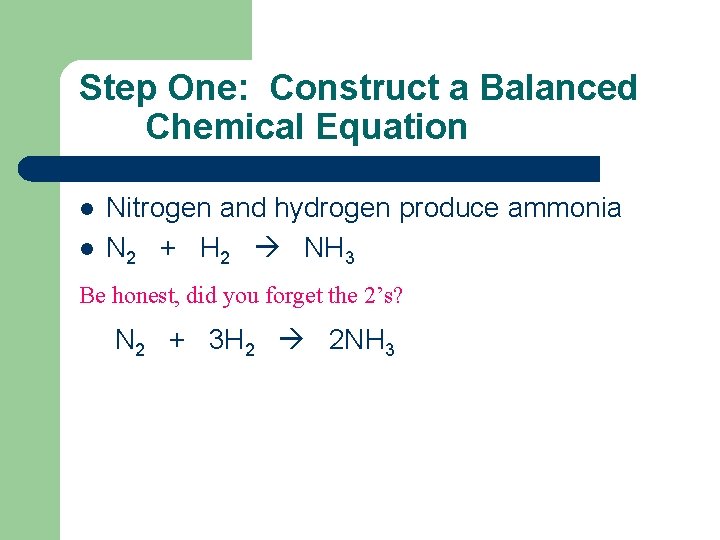
Step One: Construct a Balanced Chemical Equation l l Nitrogen and hydrogen produce ammonia N 2 + H 2 NH 3 Be honest, did you forget the 2’s? N 2 + 3 H 2 2 NH 3

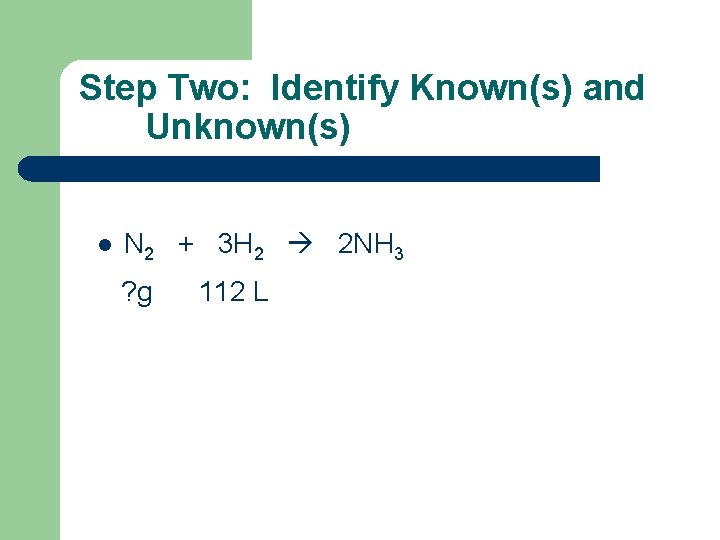
Step Two: Identify Known(s) and Unknown(s) l N 2 + 3 H 2 2 NH 3 ? g 112 L

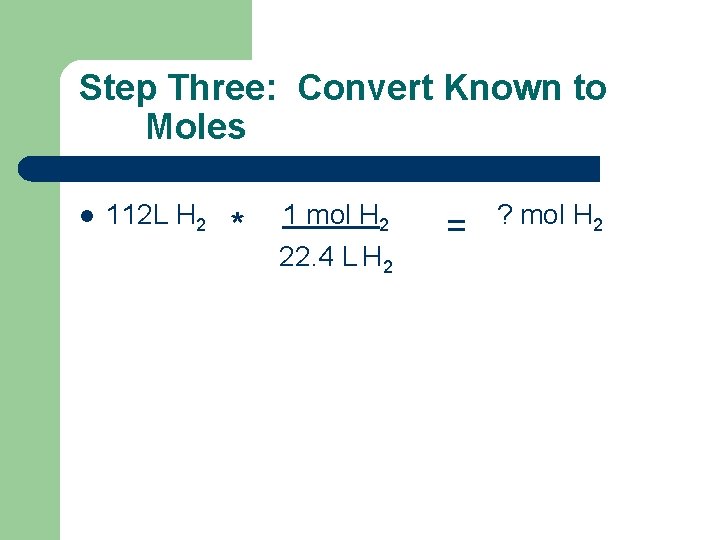
Step Three: Convert Known to Moles l 112 L H 2 * 1 mol H 2 22. 4 L H 2 = ? mol H 2

Step Three: Convert Known(s) to Moles l 112 L H 2 * 1 mol H 2 = 22. 4 L H 2 5 mol H 2

Step Four: Find Moles of Unknown(s) l l Convert moles of known to moles of unknown. Use the mole ratio from the balanced equation.

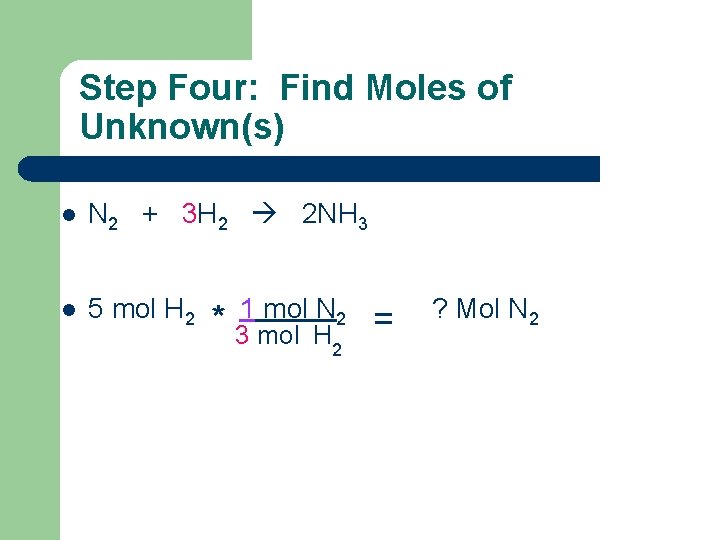
Step Four: Find Moles of Unknown(s) l N 2 + 3 H 2 2 NH 3 l 5 mol H 2 * 1 mol N 2 3 mol H 2 = ? Mol N 2

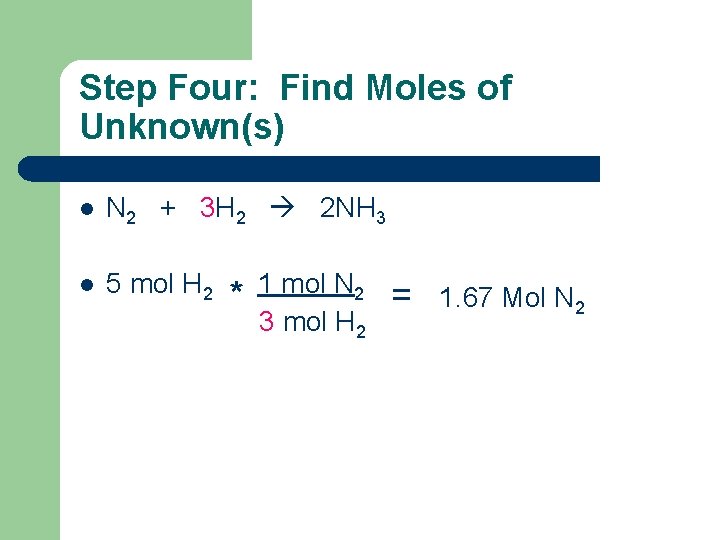
Step Four: Find Moles of Unknown(s) l N 2 + 3 H 2 2 NH 3 l 5 mol H 2 * 1 mol N 2 3 mol H 2 = 1. 67 Mol N 2

Step Five: Convert Unknown(s) into Required Units l How many grams of nitrogen? l 1. 67 Mol N 2 * 28 g N 2 1 mol N 2 = ? g N 2

Step Five: Convert Unknown(s) into Required Units l How many grams of nitrogen? l 1. 67 Mol N 2 * 28 g N 2 1 mol N 2 = 46. 76 g N 2

Your Turn…. l 560 L of oxygen will react with hydrogen to make how many grams of water?

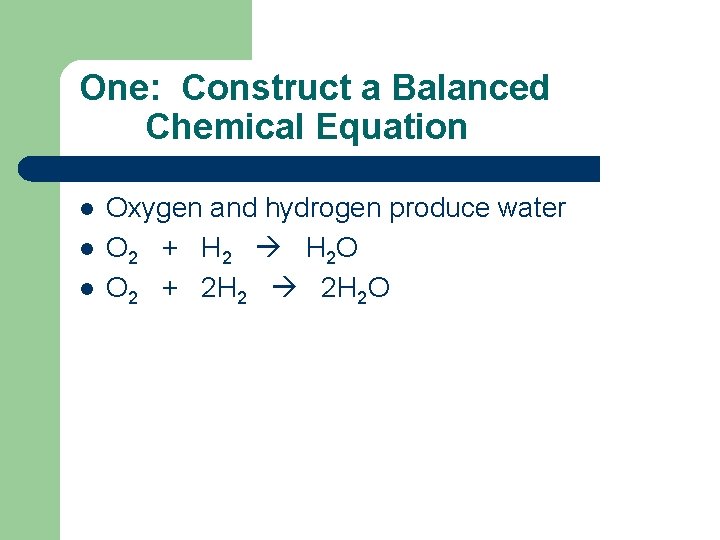
One: Construct a Balanced Chemical Equation l l l Oxygen and hydrogen produce water O 2 + H 2 O O 2 + 2 H 2 O

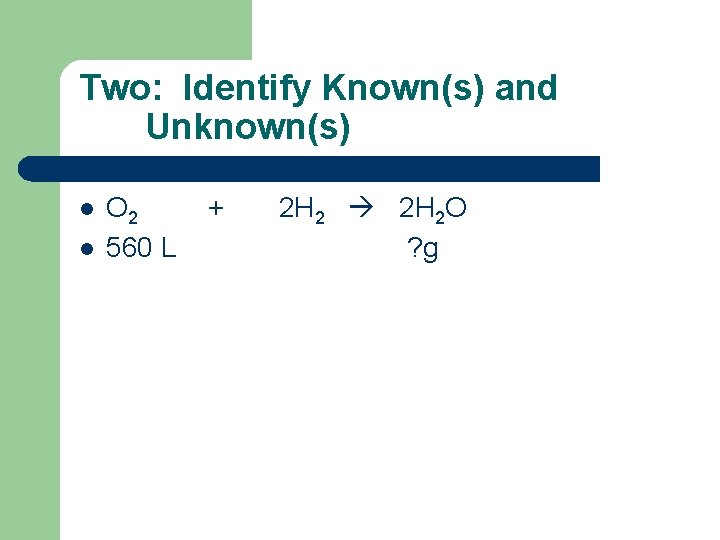
Two: Identify Known(s) and Unknown(s) l l O 2 560 L + 2 H 2 O ? g

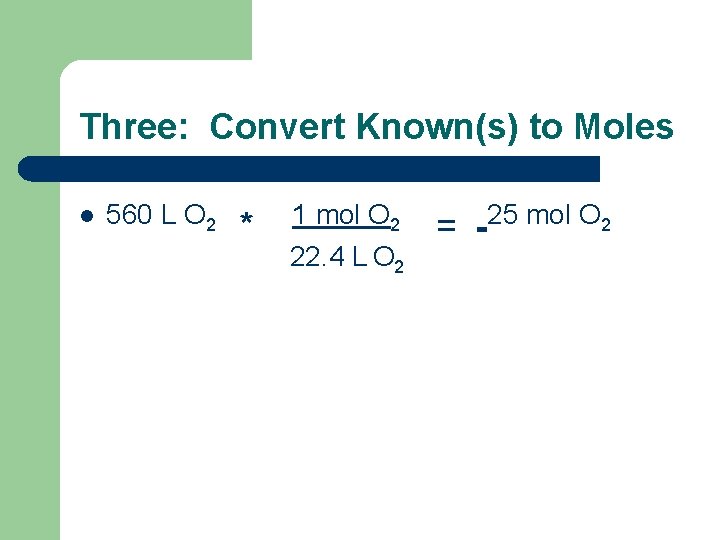
Three: Convert Known(s) to Moles l 560 L O 2 * 1 mol O 2 22. 4 L O 2 = 25 mol O 2

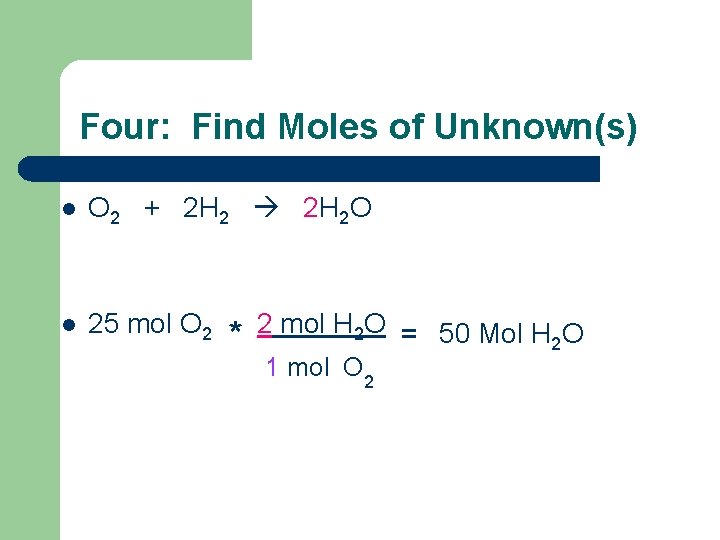
Four: Find Moles of Unknown(s) l O 2 + 2 H 2 O l 25 mol O 2 * 2 mol H 2 O = 50 Mol H O 2 1 mol O 2

Five: Convert Unknown(s) into Required Units l 50 Mol H 2 O * 18 g H 2 O = 900 g H 2 O 1 mol H 2 O

The End? ?
 Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Modern chemistry chapter 9 stoichiometry
Modern chemistry chapter 9 stoichiometry Chapter 11 stoichiometry study guide answer key
Chapter 11 stoichiometry study guide answer key Chapter 9 review stoichiometry
Chapter 9 review stoichiometry In a chemical reaction, stoichiometry refers to
In a chemical reaction, stoichiometry refers to Define reaction stoichiometry
Define reaction stoichiometry Double replacement reaction
Double replacement reaction Reaction rate and stoichiometry
Reaction rate and stoichiometry Thermite reaction formula
Thermite reaction formula General chemistry 1 stoichiometry
General chemistry 1 stoichiometry Ap chemistry stoichiometry
Ap chemistry stoichiometry Reaction rate formula
Reaction rate formula Addition reaction and substitution reaction
Addition reaction and substitution reaction Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Half life formula
Half life formula All types of reaction in chemistry
All types of reaction in chemistry A chemists shorthand way of representing chemical reaction
A chemists shorthand way of representing chemical reaction Spontaneous reaction chemistry
Spontaneous reaction chemistry Organic chemistry reaction pathways
Organic chemistry reaction pathways Endothermic reactions examples
Endothermic reactions examples Combustion reaction cartoon examples
Combustion reaction cartoon examples