STOICHIOMETRY Chapter 9 What is stoichiometry l Stoichiometry










- Slides: 16

STOICHIOMETRY Chapter 9

What is stoichiometry? l Stoichiometry is the quantitative study of reactants and products in a chemical reaction. l Another way of converting from one unit to another. A form of Dimensional Analysis.

What is a Mole? A mole is a number (or conversion unit) used to represent billions of small molecules or atoms. l One mole is equal to Avagadros number. l Avagadros number or (1 mole) = 6. 02 x 1023 particles, molecules, or atoms Ex: 1 dozen = 12 eggs A ream = 500 sheets l

How Big Is A Mole Video?

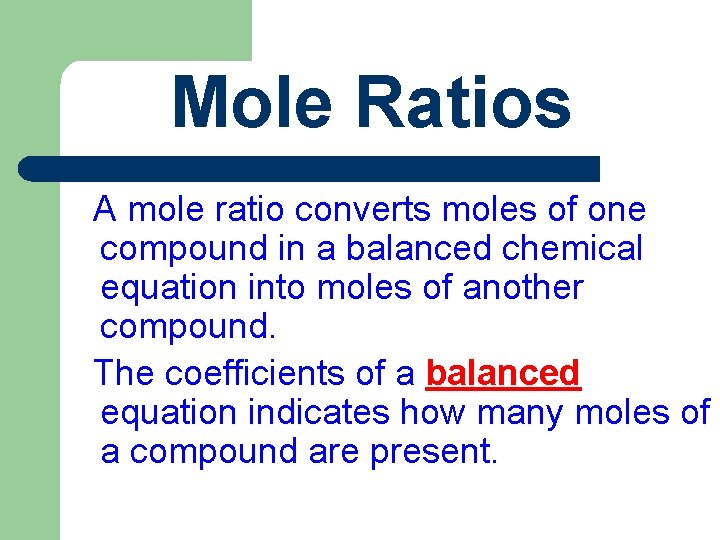
Mole Ratios A mole ratio converts moles of one compound in a balanced chemical equation into moles of another compound. The coefficients of a balanced equation indicates how many moles of a compound are present.

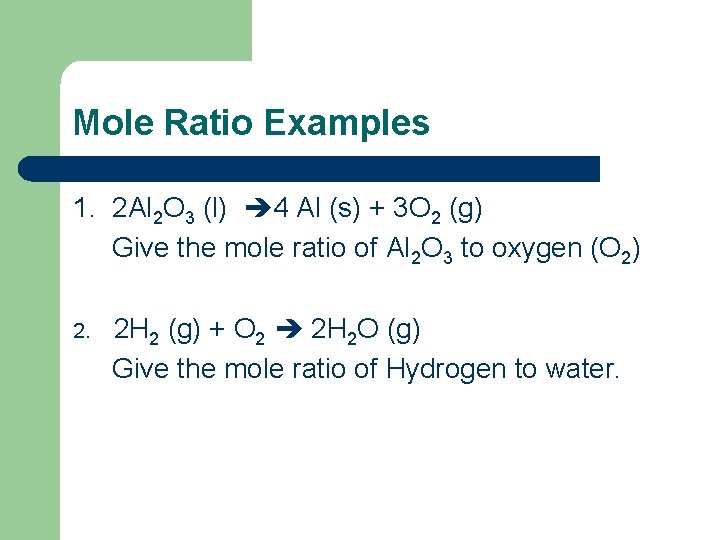
Mole Ratio Examples 1. 2 Al 2 O 3 (l) 4 Al (s) + 3 O 2 (g) Give the mole ratio of Al 2 O 3 to oxygen (O 2) 2. 2 H 2 (g) + O 2 2 H 2 O (g) Give the mole ratio of Hydrogen to water.

Answers l 1. 2: 3 l 2. 2: 2 (reduce) 1: 1

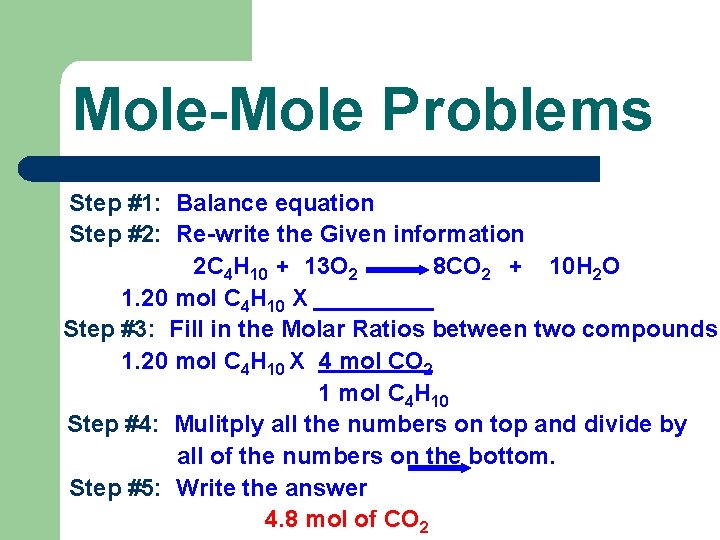
Mole- Mole Problems where the given unit is in moles and the unknown unit is in moles. l OR l Problems where the starting unit is in moles and the final unit is in moles. l

Mole-to-Mole Problems (2) A can of butane lighter fluid contains 1. 20 moles of butane (C 4 H 10). Calculate the number of moles of carbon dioxide given off when this butane is burned. *Given unit is moles and unknown is moles. Therefore; this is a mole-to – mole conversion.

Mole-Mole Problems Step #1: Balance equation Step #2: Re-write the Given information 2 C 4 H 10 + 13 O 2 8 CO 2 + 10 H 2 O 1. 20 mol C 4 H 10 X _____ Step #3: Fill in the Molar Ratios between two compounds 1. 20 mol C 4 H 10 X 4 mol CO 2 1 mol C 4 H 10 Step #4: Mulitply all the numbers on top and divide by all of the numbers on the bottom. Step #5: Write the answer 4. 8 mol of CO 2

Example 3. 0 moles of magnesium combines with oxygen to form magnesium oxide. Calculate the number of mole of magnesium oxide produced. 2 Mg (s) + O 2 (g) 2 Mg. O (s) Step #2: Re-write the Given 3. 0 mol Mg X ____ Step #4: Fill in molar ratios for two compounds 3. 0 mol Mg x 1 mol Mg. O = 3. 0 mol Mg. O 1 mol Mg

Class Assignment l Complete Ch. 9 Wksht #1 (found on my website)

Converting Moles to Grams l Problems where you are converting from moles( given unit) to mass (unknown unit). l To convert from moles to grams: 1 mole of any substance is equal to its Molar Mass

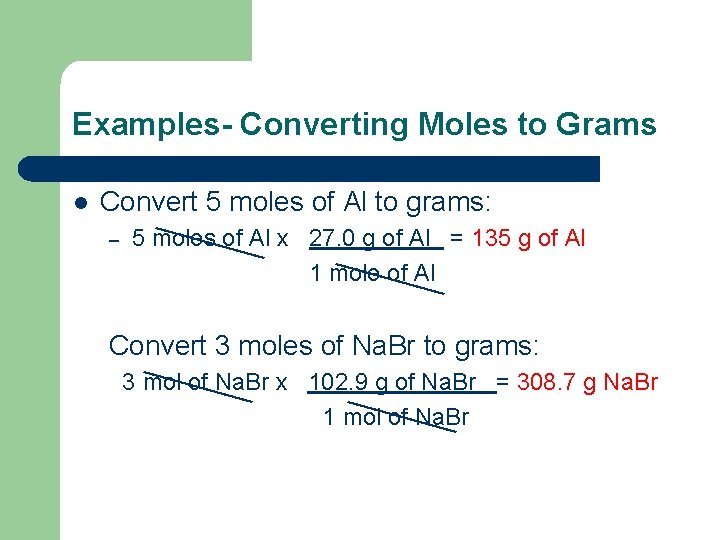
Examples- Converting Moles to Grams l Convert 5 moles of Al to grams: – 5 moles of Al x 27. 0 g of Al = 135 g of Al 1 mole of Al Convert 3 moles of Na. Br to grams: 3 mol of Na. Br x 102. 9 g of Na. Br = 308. 7 g Na. Br 1 mol of Na. Br

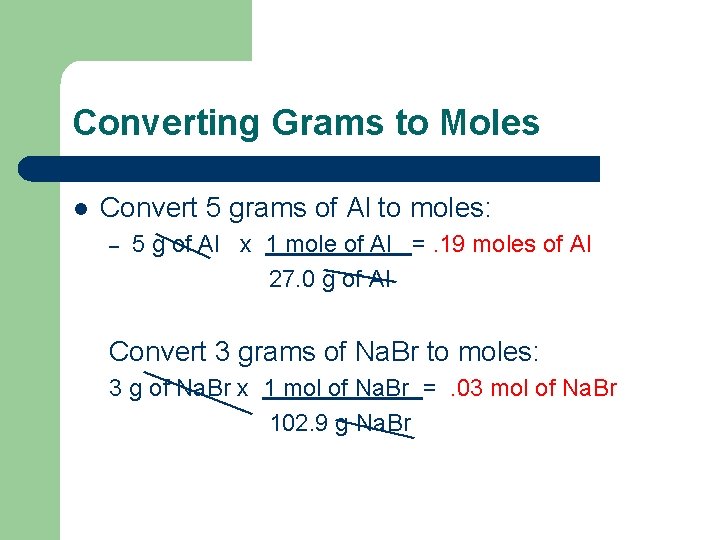
Converting Grams to Moles l Convert 5 grams of Al to moles: – 5 g of Al x 1 mole of Al =. 19 moles of Al 27. 0 g of Al Convert 3 grams of Na. Br to moles: 3 g of Na. Br x 1 mol of Na. Br =. 03 mol of Na. Br 102. 9 g Na. Br

Classwork / Homework l Complete 9. 2 Worksheet