Chapter 9 Reaction Stoichiometry 9 1 Introduction to







- Slides: 25

Chapter 9 Reaction Stoichiometry 9. 1 Introduction to Stoichiometry

Stoichiometry (STOY-KEE-AHM-EH-TREE) • The study of quantities of materials consumed and produced in chemical reactions. • Composition Stoichiometry - deals with mass relationships of elements in compounds • Reaction Stoichiometry - Involves mass relationships between reactants and products in a chemical reaction

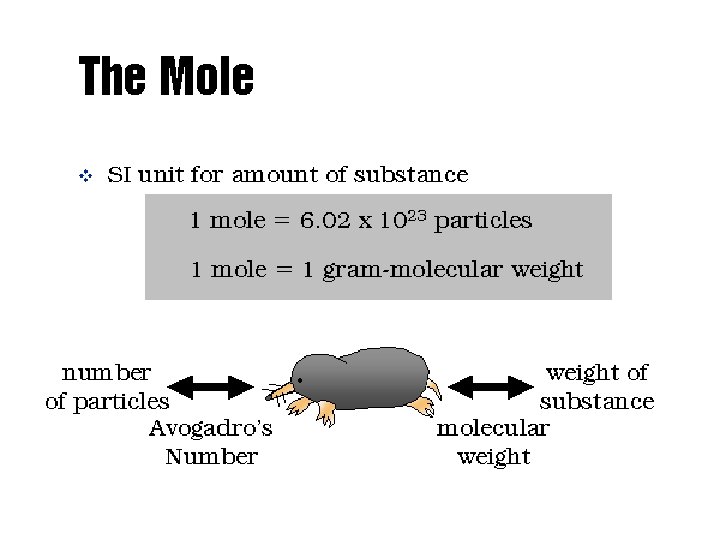
The Mole

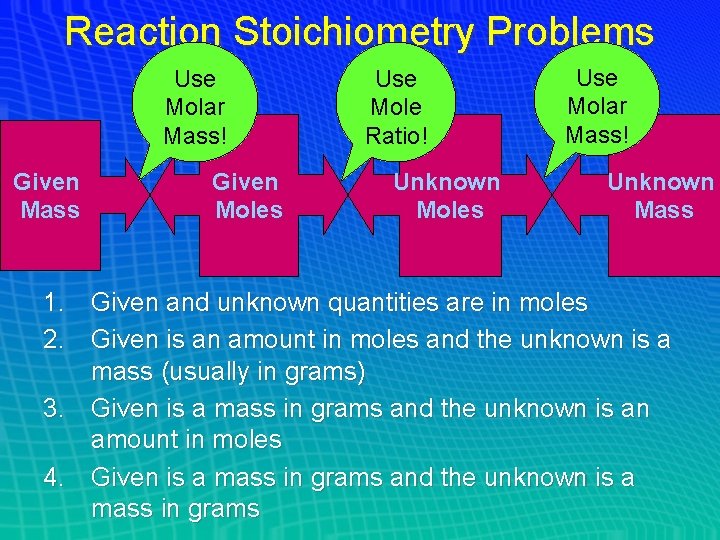
Reaction Stoichiometry Problems Use Molar Mass! Given Mass Given Moles Use Mole Ratio! Unknown Moles Use Molar Mass! Unknown Mass 1. Given and unknown quantities are in moles 2. Given is an amount in moles and the unknown is a mass (usually in grams) 3. Given is a mass in grams and the unknown is an amount in moles 4. Given is a mass in grams and the unknown is a mass in grams

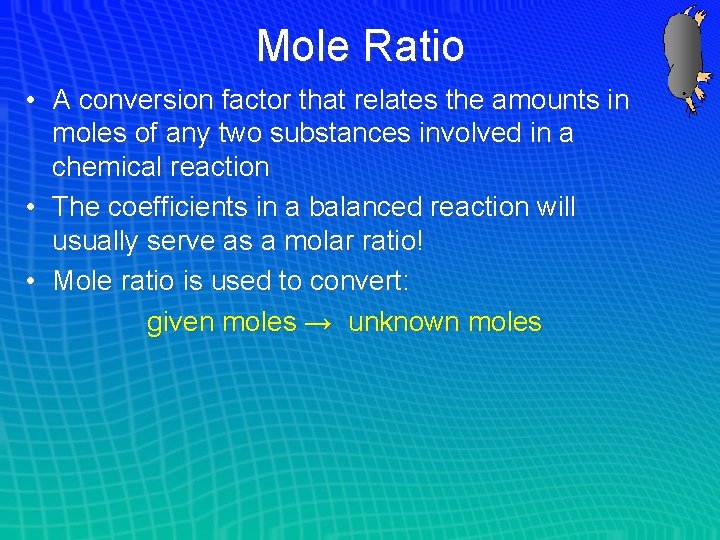
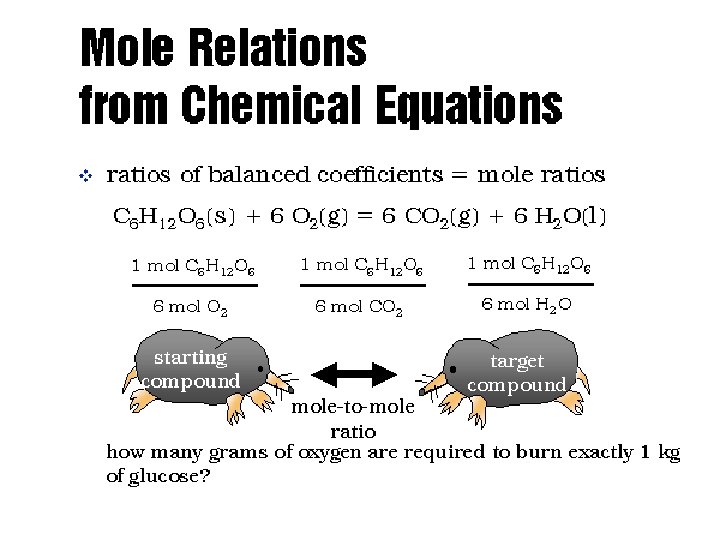
Mole Ratio • A conversion factor that relates the amounts in moles of any two substances involved in a chemical reaction • The coefficients in a balanced reaction will usually serve as a molar ratio! • Mole ratio is used to convert: given moles → unknown moles

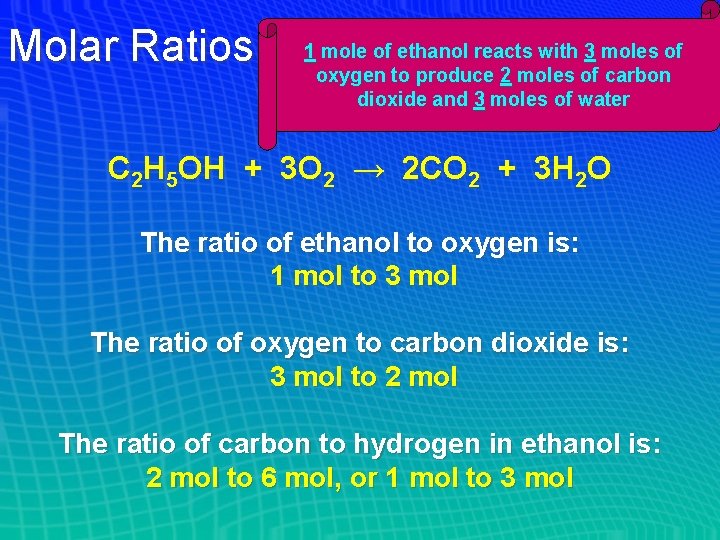
Molar Ratios 1 mole of ethanol reacts with 3 moles of oxygen to produce 2 moles of carbon dioxide and 3 moles of water C 2 H 5 OH + 3 O 2 → 2 CO 2 + 3 H 2 O The ratio of ethanol to oxygen is: 1 mol to 3 mol The ratio of oxygen to carbon dioxide is: 3 mol to 2 mol The ratio of carbon to hydrogen in ethanol is: 2 mol to 6 mol, or 1 mol to 3 mol

Mole Relations

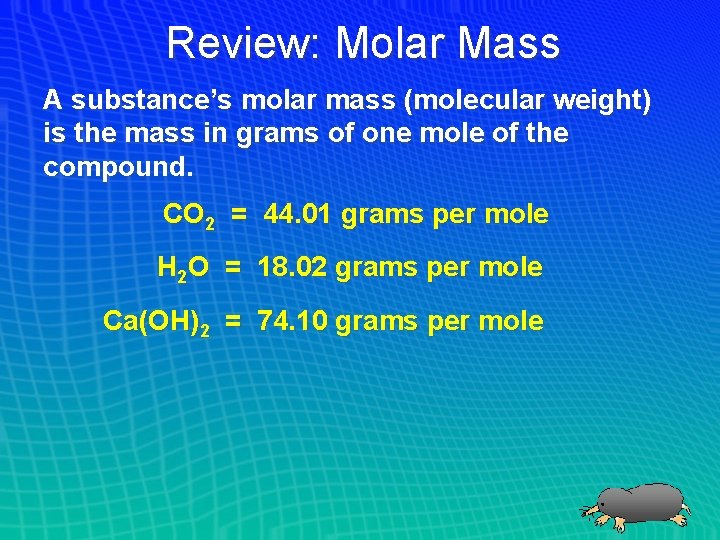
Review: Molar Mass A substance’s molar mass (molecular weight) is the mass in grams of one mole of the compound. CO 2 = 44. 01 grams per mole H 2 O = 18. 02 grams per mole Ca(OH)2 = 74. 10 grams per mole

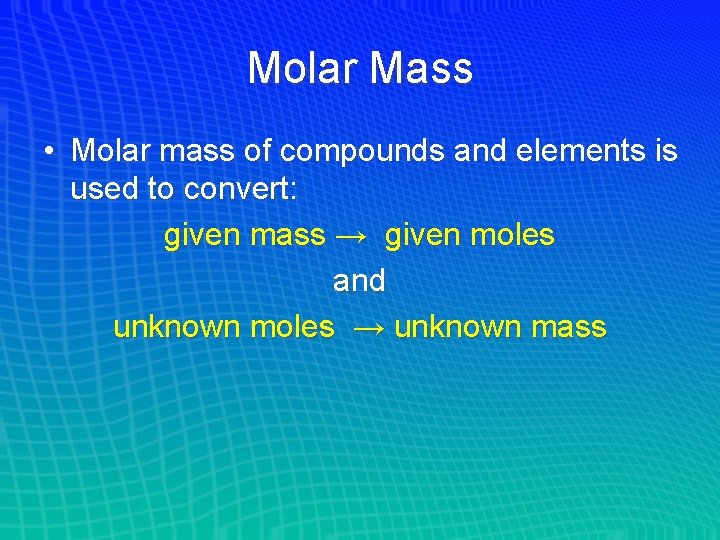
Molar Mass • Molar mass of compounds and elements is used to convert: given mass → given moles and unknown moles → unknown mass

Using Compound Masses

Chapter 9 Reaction Stoichiometry 9. 2 Ideal Stoichiometric Calculations

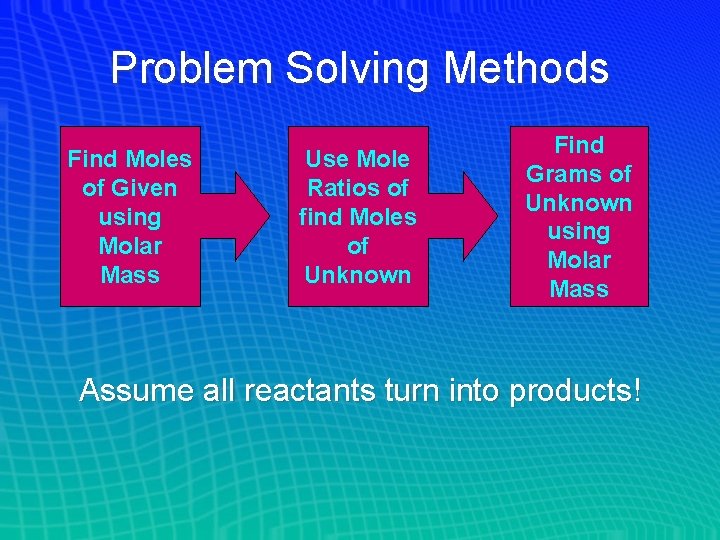
Problem Solving Methods • Ideal Stoichiometry - All reactants are converted into products • Mass-Mass Problems Start with a known mass of reactant or product, find an unknown mass of another reactant or product • All other stoichiometry problems are derivations (shortened versions) of this larger solution

Problem Solving Methods Find Moles of Given using Molar Mass Use Mole Ratios of find Moles of Unknown Find Grams of Unknown using Molar Mass Assume all reactants turn into products!

Steps to Solving Problems 1. Start with a correctly balanced chemical equation. 2. Use key words in the problem statement to identify substances as either reactants or products. 3. Determine what units you've been given and what you are being asked to find. 4. Label each step with the correct units! 5. The units from the numerator of the first step become the units in the denominator of the next step, and so forth. 6. Stop when you have an answer with the units that you are searching for!

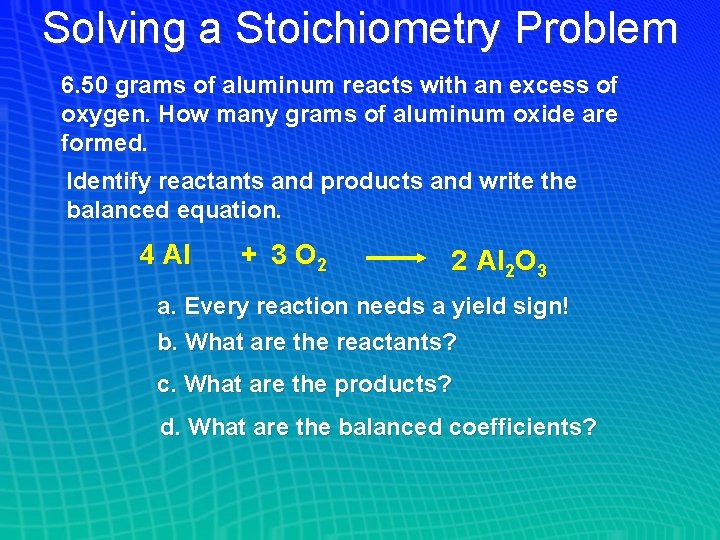
Solving a Stoichiometry Problem 6. 50 grams of aluminum reacts with an excess of oxygen. How many grams of aluminum oxide are formed. Identify reactants and products and write the balanced equation. 4 Al + 3 O 2 2 Al 2 O 3 a. Every reaction needs a yield sign! b. What are the reactants? c. What are the products? d. What are the balanced coefficients?

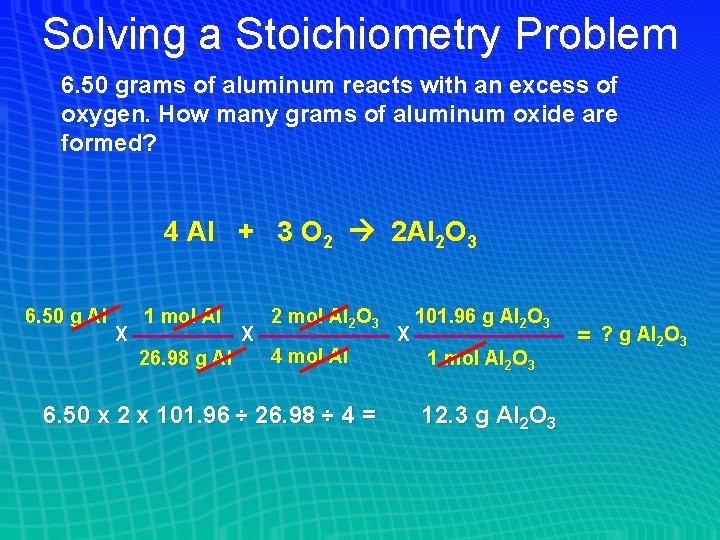
Solving a Stoichiometry Problem 6. 50 grams of aluminum reacts with an excess of oxygen. How many grams of aluminum oxide are formed? 4 Al + 3 O 2 2 Al 2 O 3 6. 50 g Al X 1 mol Al 26. 98 g Al X 2 mol Al 2 O 3 4 mol Al 6. 50 x 2 x 101. 96 ÷ 26. 98 ÷ 4 = X 101. 96 g Al 2 O 3 1 mol Al 2 O 3 12. 3 g Al 2 O 3 = ? g Al 2 O 3

Chapter 9 Reaction Stoichiometry 9. 3 Limiting Reactants & Percent Yield

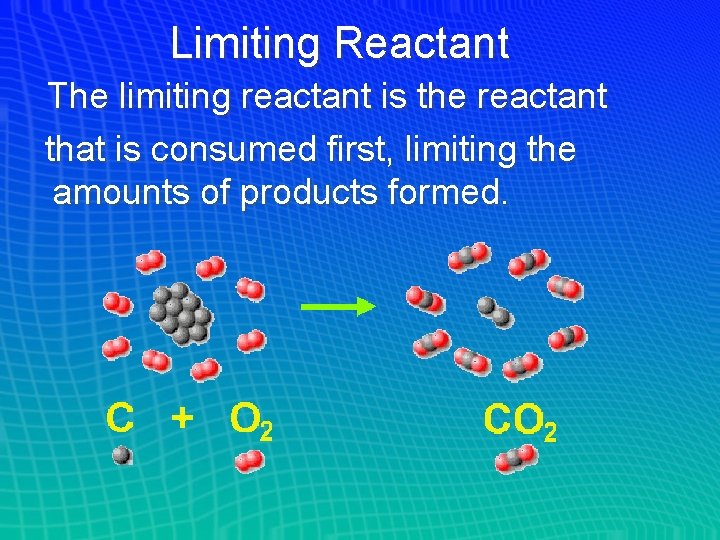
Limiting Reactant The limiting reactant is the reactant that is consumed first, limiting the amounts of products formed.

Limiting Reactant? • • • I want to make chocolate chip cookies. I find in my kitchen: 40 lbs. of butter two lbs. of salt 1 gallon of vanilla extract 80 lbs. of chocolate chips 200 lbs. of flour 150 lbs. of sugar 150 lbs. of brown sugar ten lbs. of baking soda TWO eggs It should be clear that the number of cookies I make will be limited by the number of eggs!

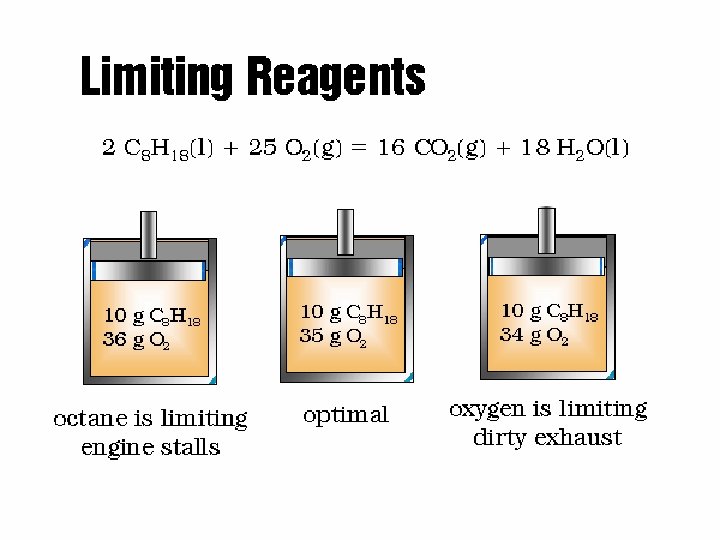
Limiting Reagents - Combustion

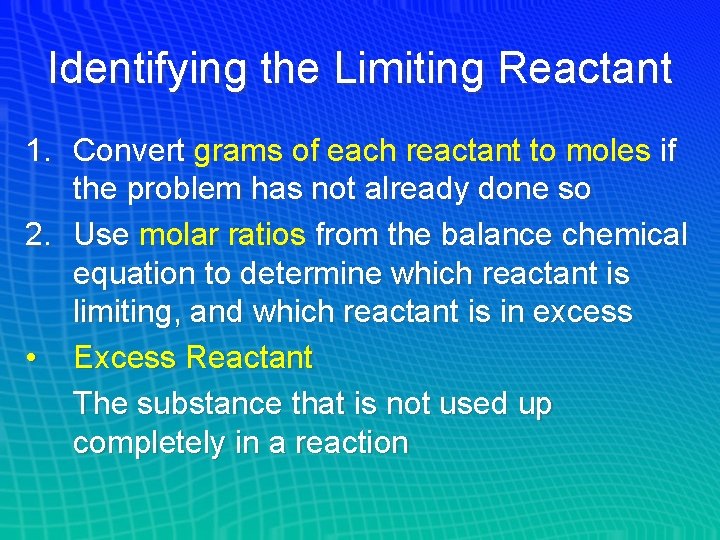
Identifying the Limiting Reactant 1. Convert grams of each reactant to moles if the problem has not already done so 2. Use molar ratios from the balance chemical equation to determine which reactant is limiting, and which reactant is in excess • Excess Reactant The substance that is not used up completely in a reaction

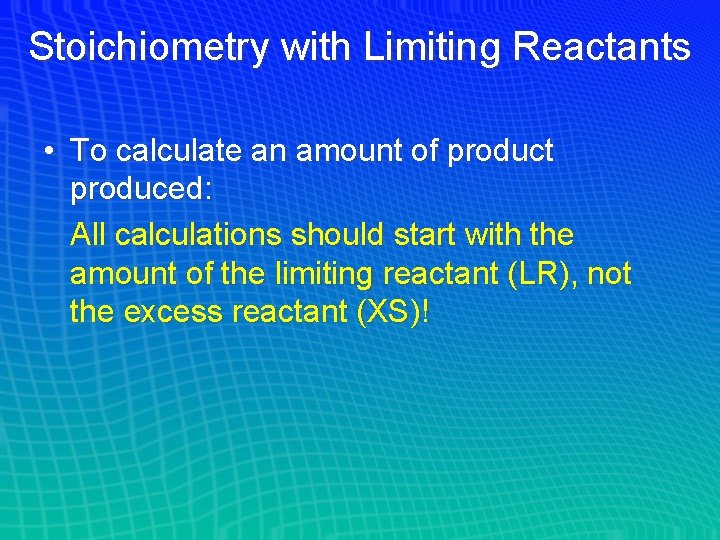
Stoichiometry with Limiting Reactants • To calculate an amount of product produced: All calculations should start with the amount of the limiting reactant (LR), not the excess reactant (XS)!

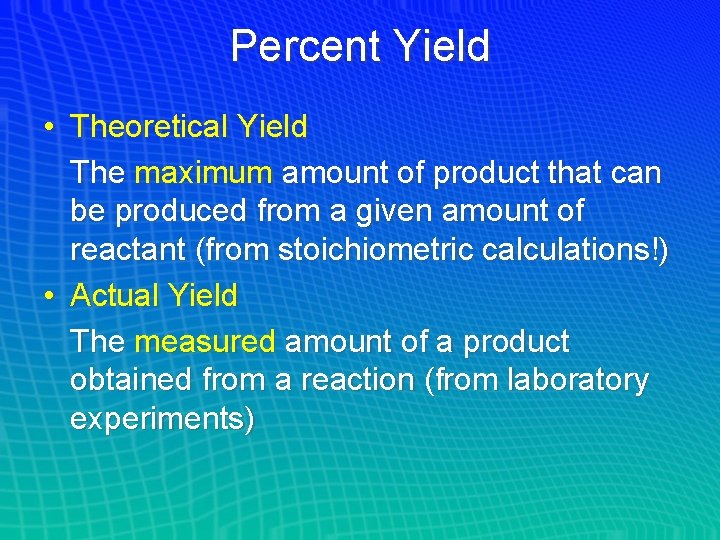
Percent Yield • Theoretical Yield The maximum amount of product that can be produced from a given amount of reactant (from stoichiometric calculations!) • Actual Yield The measured amount of a product obtained from a reaction (from laboratory experiments)

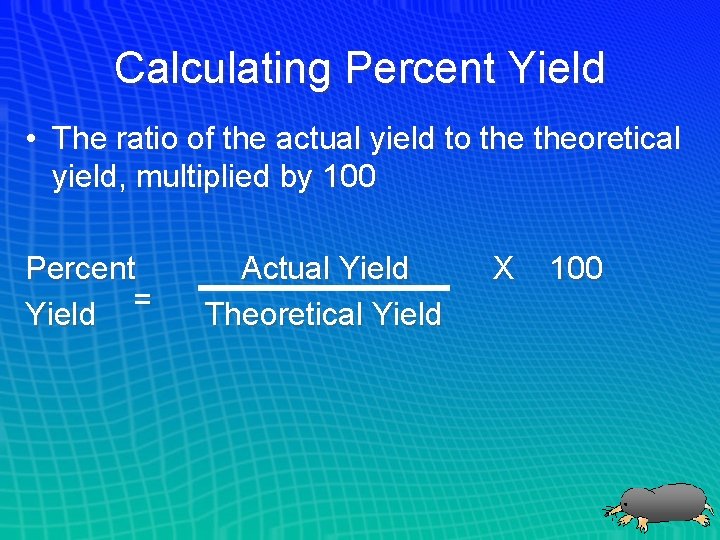
Calculating Percent Yield • The ratio of the actual yield to theoretical yield, multiplied by 100 Percent = Yield Actual Yield Theoretical Yield X 100

How many times did you see the mole? ? ? 8