Ch 11 Stoichiometry Sec 11 4 Percent Yield







- Slides: 12

Ch. 11: Stoichiometry Sec. 11. 4: Percent Yield

Objectives n n Calculate theoretical yield of a chemical reaction from data. Determine the percent yield for a chemical reaction.

Percent Yield Suppose you were practicing jump shots. In your best practice, you shot 75 balls. THEORETICALLY, you should have been successful 75 times. But, in ACTUALITY, you shot 49 out of 75. To determine your efficiency, you calculate a percentage: actual shots x 100 theoretical shots = 65%

Percent Yield n n Similar calculations are made to determine the success of chemical reactions. Most reactions never succeed in producing the predicted (theoretical) amount of product. Why? n n Not every reaction goes to completion. If liquids are produced, they may adhere to glassware. If gases are produced, they may evaporate off. Solids can be lost on transfer from one container to another. Sometimes side reactions occur, reducing the yield of the intended product.

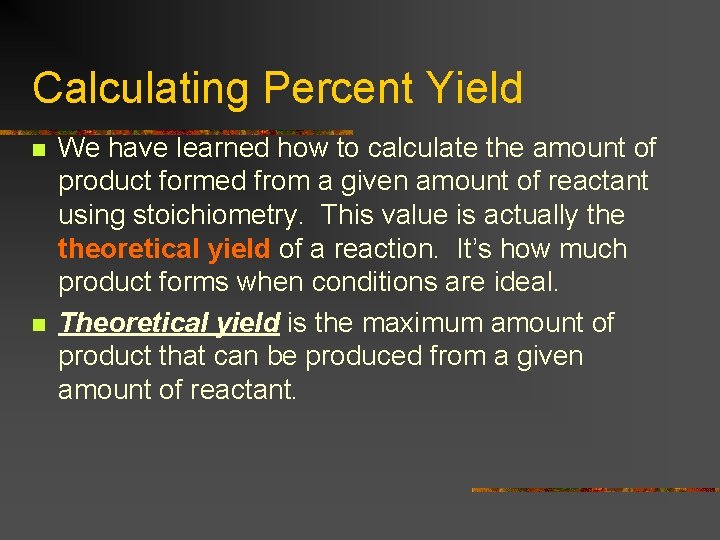
Calculating Percent Yield n n We have learned how to calculate the amount of product formed from a given amount of reactant using stoichiometry. This value is actually theoretical yield of a reaction. It’s how much product forms when conditions are ideal. Theoretical yield is the maximum amount of product that can be produced from a given amount of reactant.

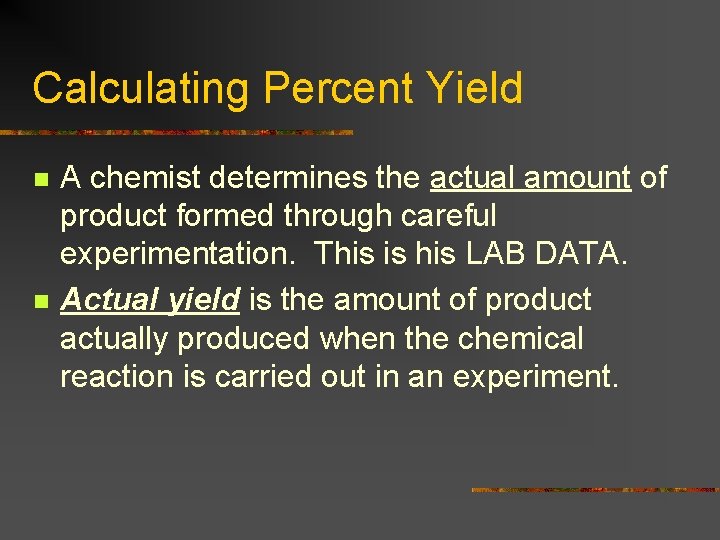
Calculating Percent Yield n n A chemist determines the actual amount of product formed through careful experimentation. This is his LAB DATA. Actual yield is the amount of product actually produced when the chemical reaction is carried out in an experiment.

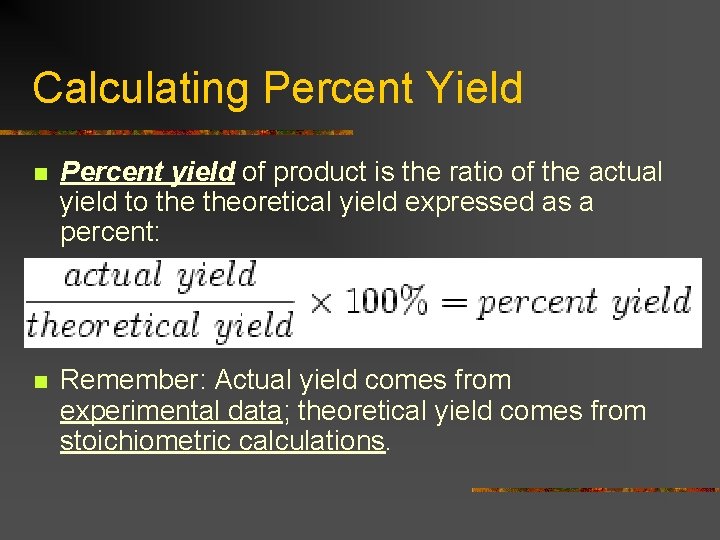
Calculating Percent Yield n Percent yield of product is the ratio of the actual yield to theoretical yield expressed as a percent: n Remember: Actual yield comes from experimental data; theoretical yield comes from stoichiometric calculations.

Sample Problem n When potassium chromate (K 2 Cr. O 4) is added to a solution containing 0. 500 g silver nitrate (Ag. NO 3), solid silver chromate (Ag 2 Cr. O 4) is formed. n n Determine theoretical yield of silver chromate precipitate. If 0. 455 g of silver chromate is obtained, calculate the percent yield.

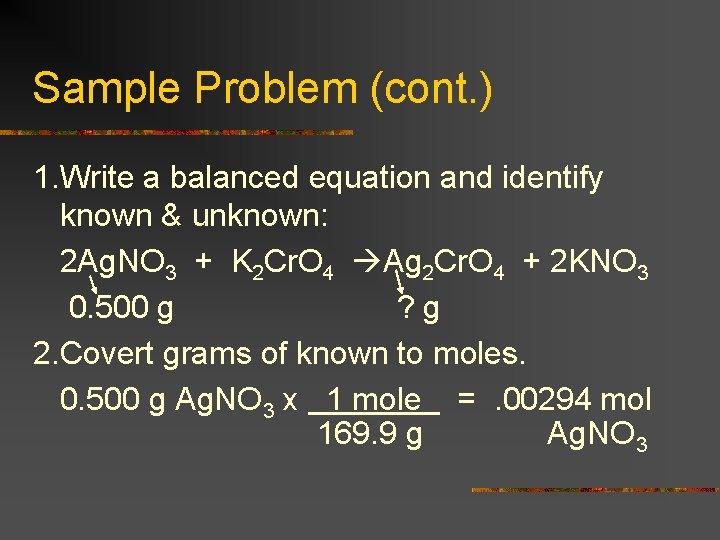
Sample Problem (cont. ) 1. Write a balanced equation and identify known & unknown: 2 Ag. NO 3 + K 2 Cr. O 4 Ag 2 Cr. O 4 + 2 KNO 3 0. 500 g ? g 2. Covert grams of known to moles. 0. 500 g Ag. NO 3 x 1 mole =. 00294 mol 169. 9 g Ag. NO 3

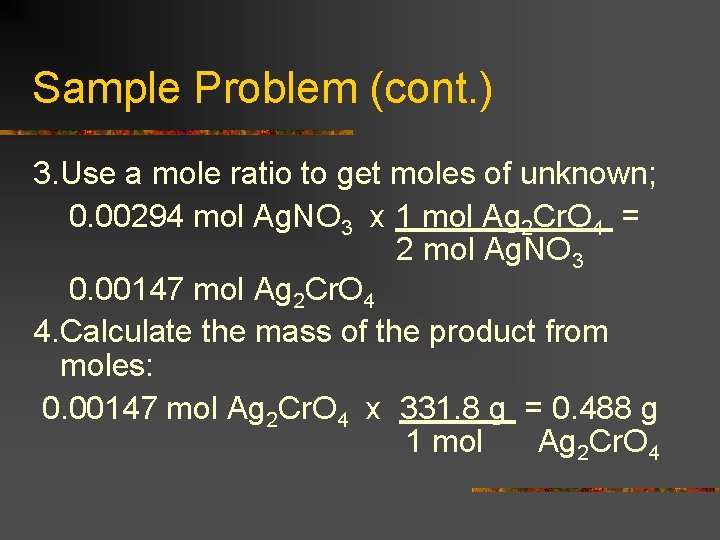
Sample Problem (cont. ) 3. Use a mole ratio to get moles of unknown; 0. 00294 mol Ag. NO 3 x 1 mol Ag 2 Cr. O 4 = 2 mol Ag. NO 3 0. 00147 mol Ag 2 Cr. O 4 4. Calculate the mass of the product from moles: 0. 00147 mol Ag 2 Cr. O 4 x 331. 8 g = 0. 488 g 1 mol Ag 2 Cr. O 4

Sample Problem (cont. ) q This is theoretical yield: 0. 488 g Ag 2 Cr. O 4 To get the percent yield, divide the actual yield (given in the problem as 0. 455 g) by theoretical yield & multiply by 100: 0. 455 g x 100 = 93. 2% yield of Ag 2 Cr. O 4 0. 488 g NOTE: Percent yield will NEVER be greater than 100%.

Practice Problems n q If 14. 0 g Al(OH)3 is present in an antacid tablet, determine theoretical yield of Al. Cl 3 produced when the tablet reacts with an excess of stomach acid. If the actual yield is 22. 0 g, what is the percent yield? Al(OH)3 + 3 HCl Al. Cl 3 + 3 H 2 O Zinc reacts with iodine in a synthesis reaction. Write a balanced chemical equation for the reaction. Determine theoretical yield if a 125 g of zinc were used. Determine the percent yield if 116 g of product was recovered.