Single Replacement Reactions 1 There are 5 major








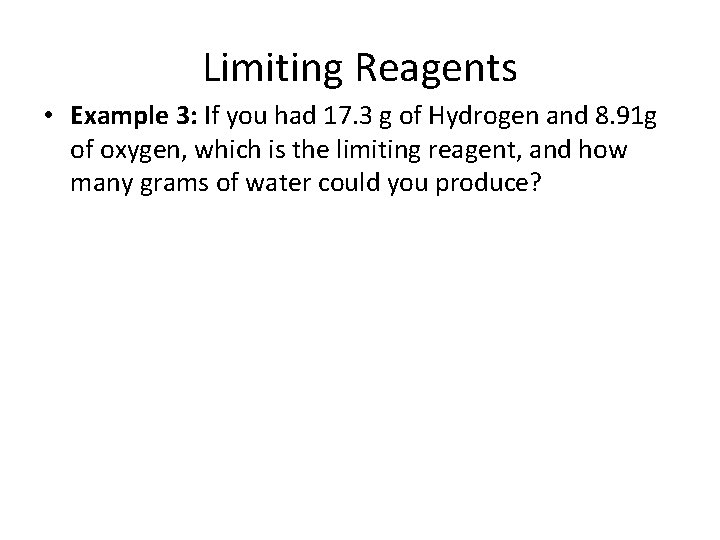
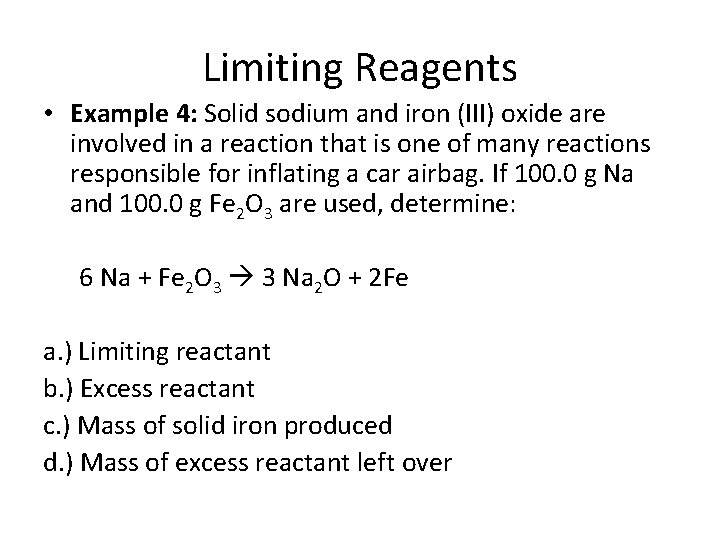

- Slides: 42

Single Replacement Reactions • 1. ) There are 5 major types of chemical reactions – A. Combustion: burns, explodes – B. Synthesis: to make something new – C. Decomposition: break down – D. Double Replacement: 2 elements change – E. Single Replacement: 1 element changes

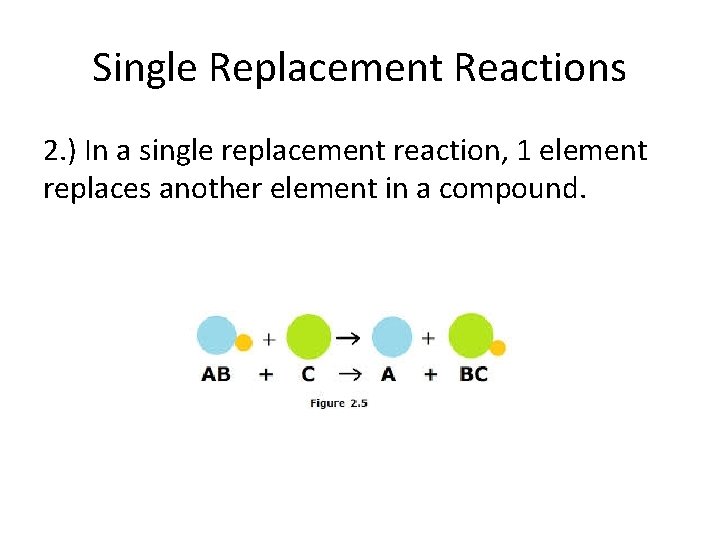
Single Replacement Reactions 2. ) In a single replacement reaction, 1 element replaces another element in a compound.

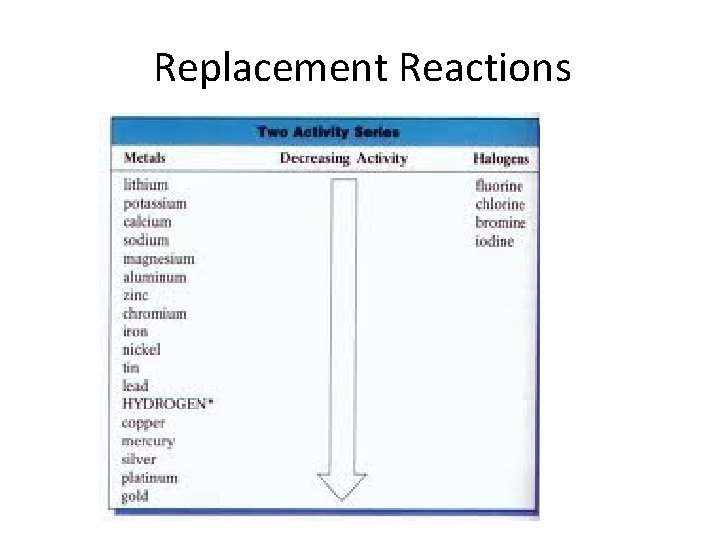
Replacement Reactions 3. ) Not all metals can be replaced, we use the reactivity series of metals list to predict changes. 4. ) The higher an element is on the list, the more likely it is to replace another element.

Replacement Reactions

Single Replacement Reactions Example 1 : Zn + Ag(NO 3) Zn (No 3) + Ag - Zinc is higher on the list than Silver, therefore it can switch places.

Single Replacement Reactions Example 2 : Au + Ni(SO 4) No reaction - Gold is NOT higher on the list than Nickel, therefore it cannot switch places.

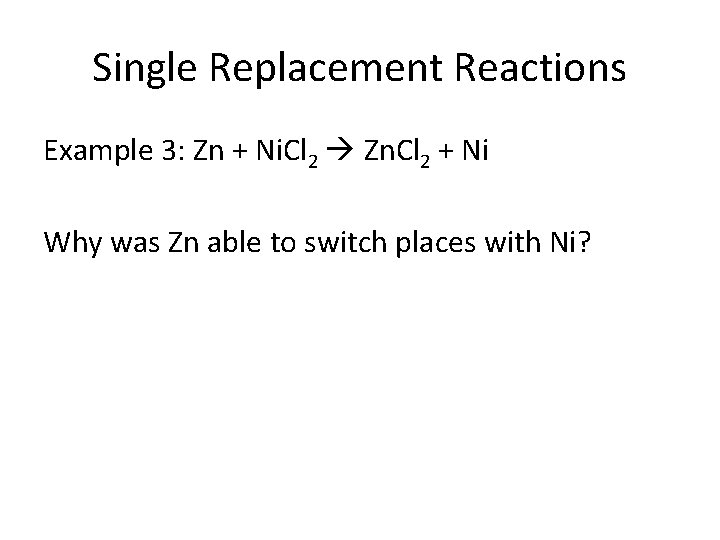
Single Replacement Reactions Example 3: Zn + Ni. Cl 2 Zn. Cl 2 + Ni Why was Zn able to switch places with Ni?

Single Replacement Reactions Example 4: Fe + Mg. Cl 2 No reaction Why did the reaction not occur?

Notes. Advanced Stoichiometry with Moles

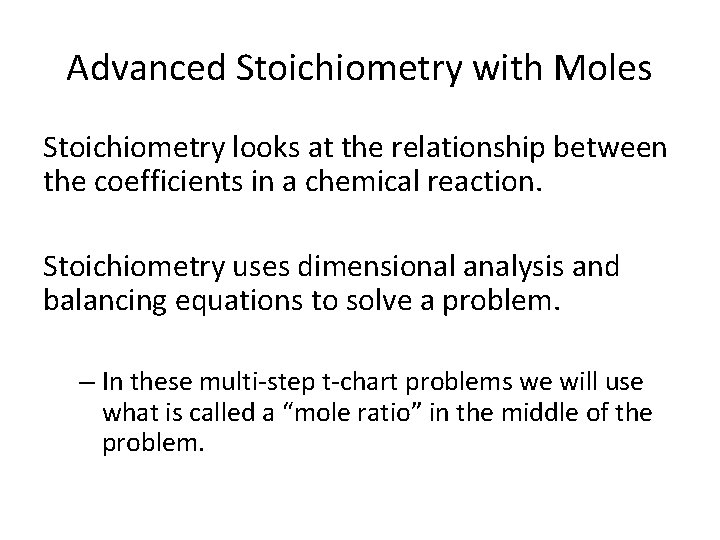
Advanced Stoichiometry with Moles Stoichiometry looks at the relationship between the coefficients in a chemical reaction. Stoichiometry uses dimensional analysis and balancing equations to solve a problem. – In these multi-step t-chart problems we will use what is called a “mole ratio” in the middle of the problem.

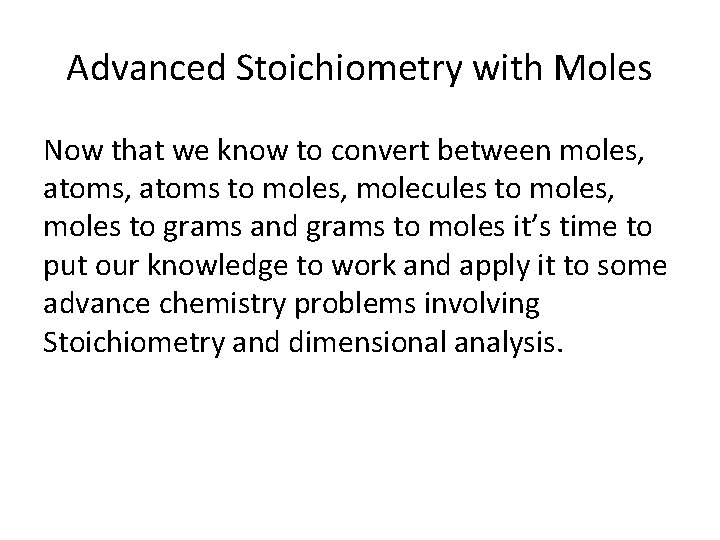
Advanced Stoichiometry with Moles Now that we know to convert between moles, atoms, atoms to moles, molecules to moles, moles to grams and grams to moles it’s time to put our knowledge to work and apply it to some advance chemistry problems involving Stoichiometry and dimensional analysis.

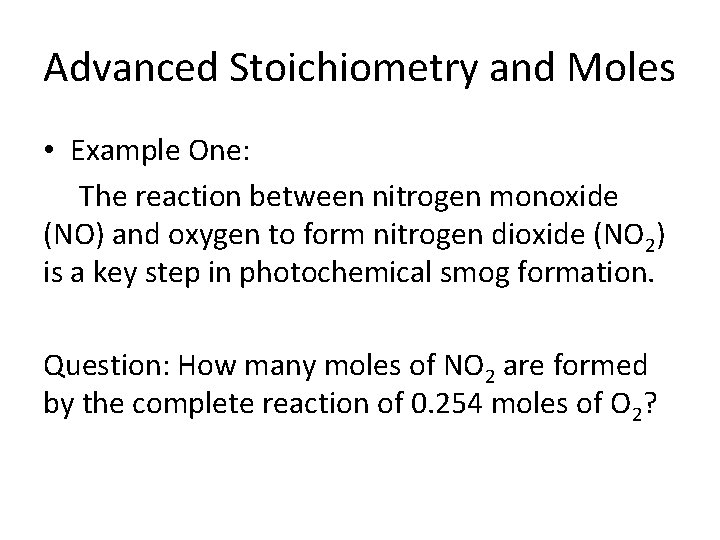
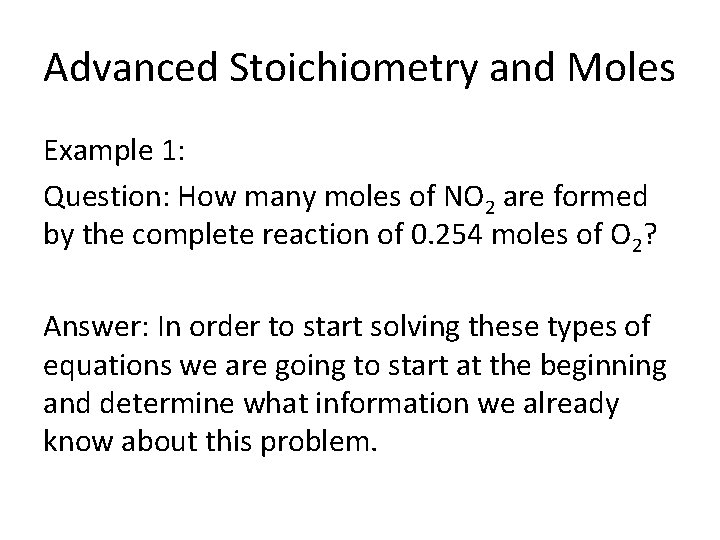
Advanced Stoichiometry and Moles • Example One: The reaction between nitrogen monoxide (NO) and oxygen to form nitrogen dioxide (NO 2) is a key step in photochemical smog formation. Question: How many moles of NO 2 are formed by the complete reaction of 0. 254 moles of O 2?

Advanced Stoichiometry and Moles Example 1: Question: How many moles of NO 2 are formed by the complete reaction of 0. 254 moles of O 2? Answer: In order to start solving these types of equations we are going to start at the beginning and determine what information we already know about this problem.

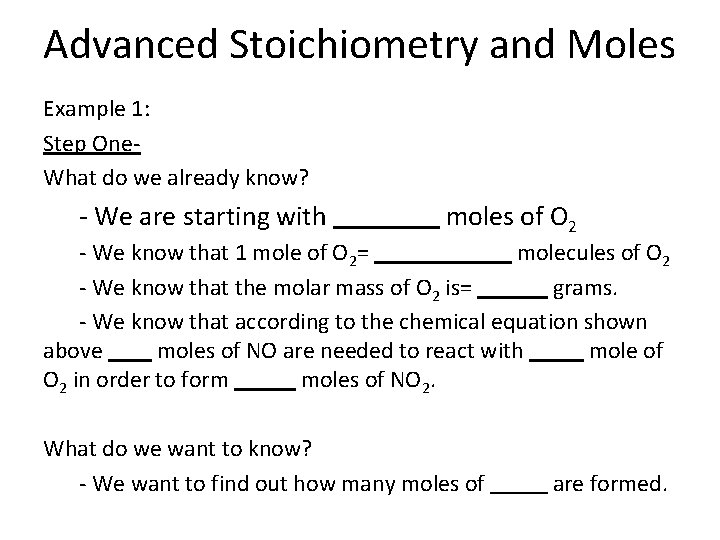
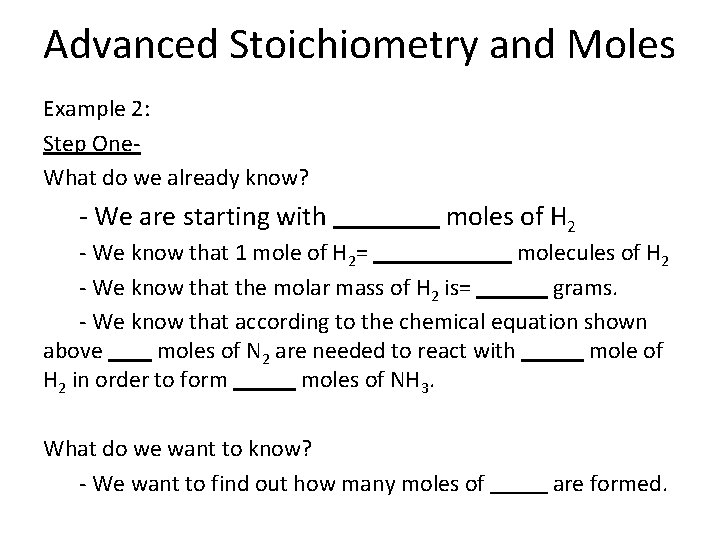
Advanced Stoichiometry and Moles Example 1: Step One- What do we already know? - We are starting with moles of O 2 - We know that 1 mole of O 2= molecules of O 2 - We know that the molar mass of O 2 is= grams. - We know that according to the chemical equation shown above moles of NO are needed to react with mole of O 2 in order to form moles of NO 2. What do we want to know? - We want to find out how many moles of are formed.

Advanced Stoichiometry and Moles Example 1: Step Two- Set-up the Dimensional Analysis Problem using the steps we have discussed before:

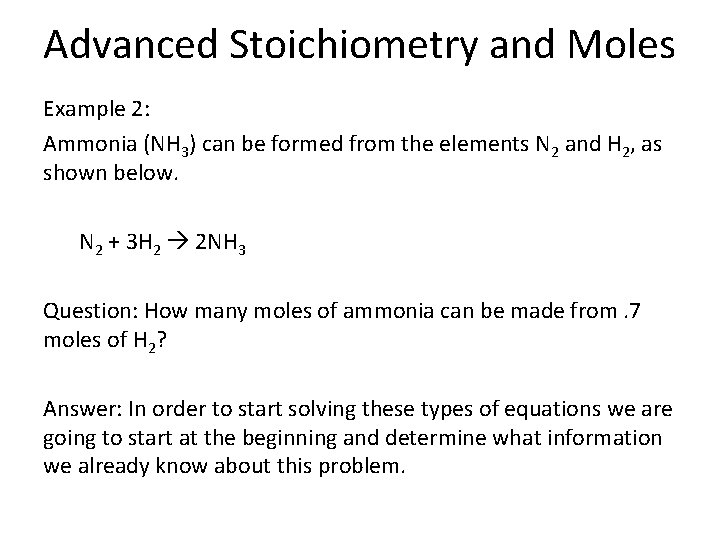
Advanced Stoichiometry and Moles Example 2: Ammonia (NH 3) can be formed from the elements N 2 and H 2, as shown below. N 2 + 3 H 2 2 NH 3 Question: How many moles of ammonia can be made from. 7 moles of H 2? Answer: In order to start solving these types of equations we are going to start at the beginning and determine what information we already know about this problem.

Advanced Stoichiometry and Moles Example 2: Step One- What do we already know? - We are starting with moles of H 2 - We know that 1 mole of H 2= molecules of H 2 - We know that the molar mass of H 2 is= grams. - We know that according to the chemical equation shown above moles of N 2 are needed to react with mole of H 2 in order to form moles of NH 3. What do we want to know? - We want to find out how many moles of are formed.

Advanced Stoichiometry and Moles Example 2: Step Two- Set-up the Dimensional Analysis Problem using the steps we have discussed before:

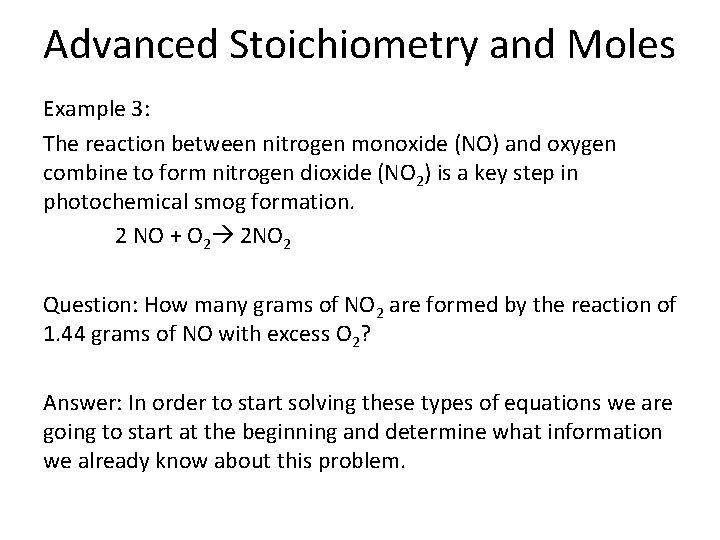
Advanced Stoichiometry and Moles Example 3: The reaction between nitrogen monoxide (NO) and oxygen combine to form nitrogen dioxide (NO 2) is a key step in photochemical smog formation. 2 NO + O 2 2 NO 2 Question: How many grams of NO 2 are formed by the reaction of 1. 44 grams of NO with excess O 2? Answer: In order to start solving these types of equations we are going to start at the beginning and determine what information we already know about this problem.

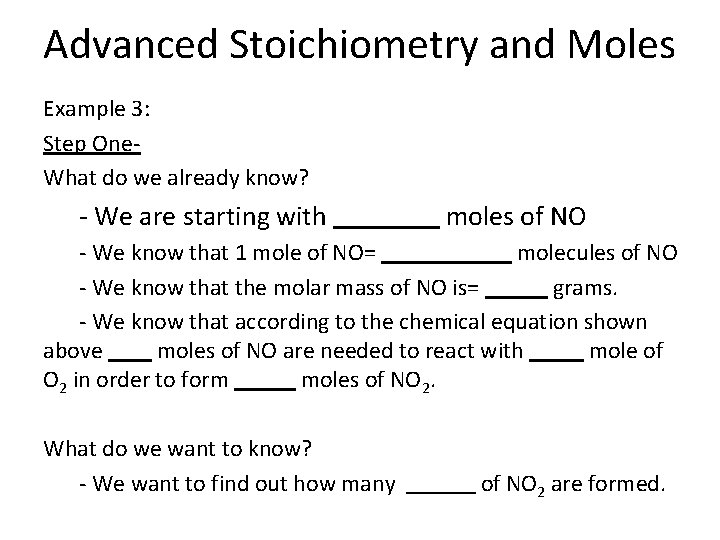
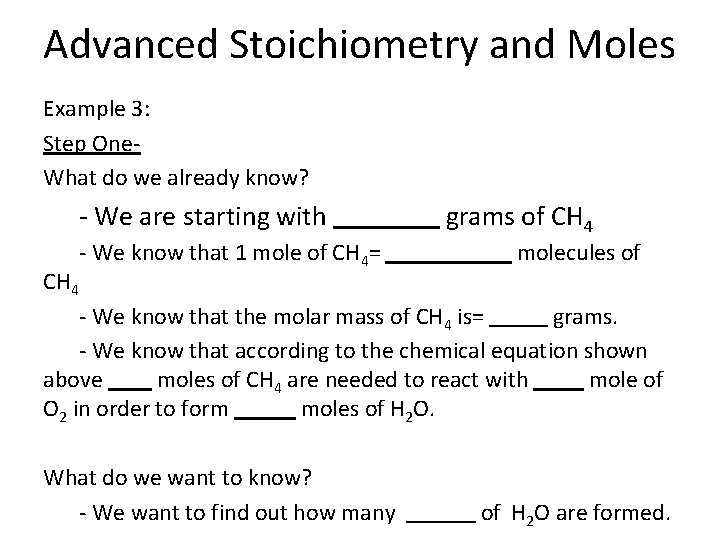
Advanced Stoichiometry and Moles Example 3: Step One- What do we already know? - We are starting with moles of NO - We know that 1 mole of NO= molecules of NO - We know that the molar mass of NO is= grams. - We know that according to the chemical equation shown above moles of NO are needed to react with mole of O 2 in order to form moles of NO 2. What do we want to know? - We want to find out how many of NO 2 are formed.

Advanced Stoichiometry and Moles Example 3: Step Two- Set-up the Dimensional Analysis Problem using the steps we have discussed before:

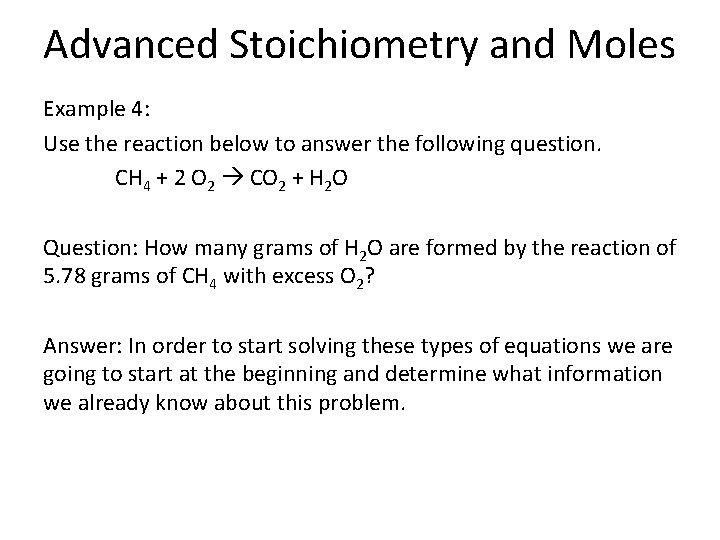
Advanced Stoichiometry and Moles Example 4: Use the reaction below to answer the following question. CH 4 + 2 O 2 CO 2 + H 2 O Question: How many grams of H 2 O are formed by the reaction of 5. 78 grams of CH 4 with excess O 2? Answer: In order to start solving these types of equations we are going to start at the beginning and determine what information we already know about this problem.

Advanced Stoichiometry and Moles Example 3: Step One- What do we already know? - We are starting with CH 4 - We know that 1 mole of CH 4= grams of CH 4 molecules of - We know that the molar mass of CH 4 is= grams. - We know that according to the chemical equation shown above moles of CH 4 are needed to react with mole of O 2 in order to form moles of H 2 O. What do we want to know? - We want to find out how many of H 2 O are formed.

Advanced Stoichiometry and Moles Example 4: Step Two- Set-up the Dimensional Analysis Problem using the steps we have discussed before:

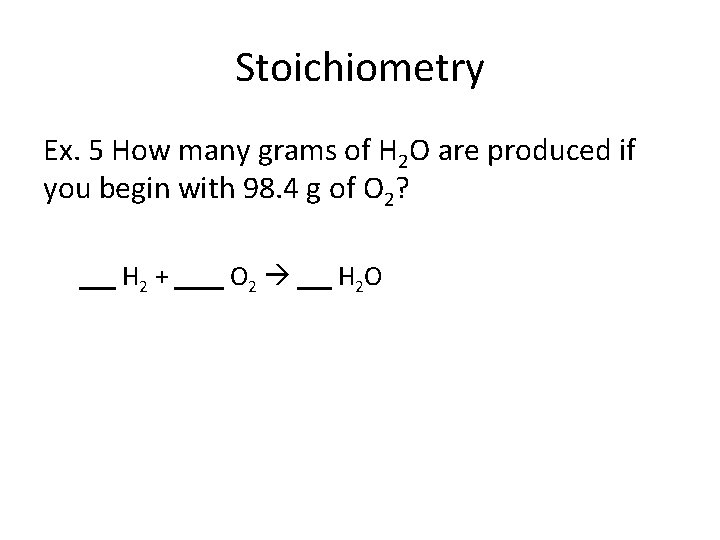
Stoichiometry Ex. 5 How many grams of H 2 O are produced if you begin with 98. 4 g of O 2? H 2 + O 2 H 2 O

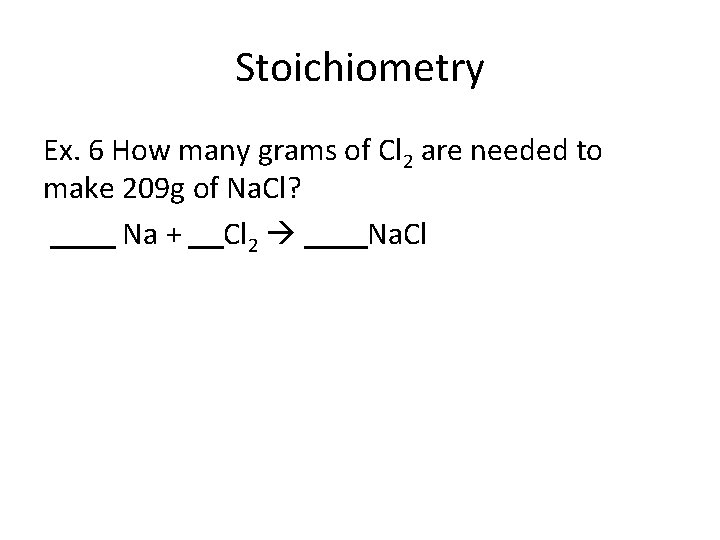
Stoichiometry Ex. 6 How many grams of Cl 2 are needed to make 209 g of Na. Cl? Na + Cl 2 Na. Cl

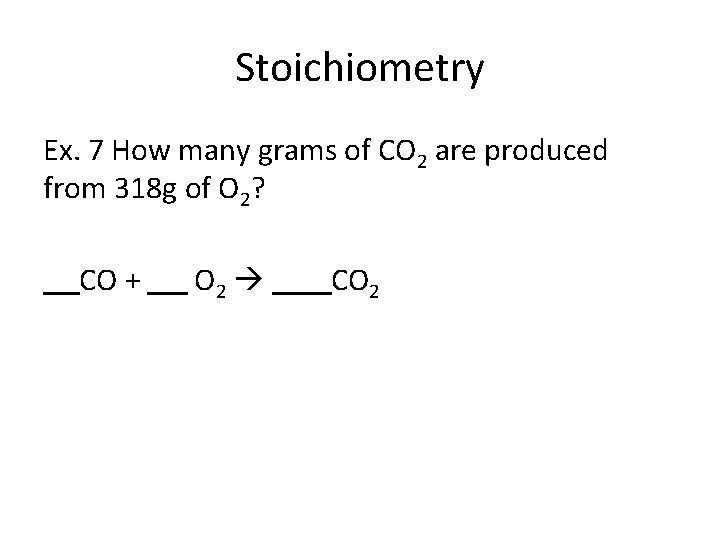
Stoichiometry Ex. 7 How many grams of CO 2 are produced from 318 g of O 2? CO + O 2 CO 2

Notes. Limiting Reagent

Limiting Reagents • What is a limiting reagent?

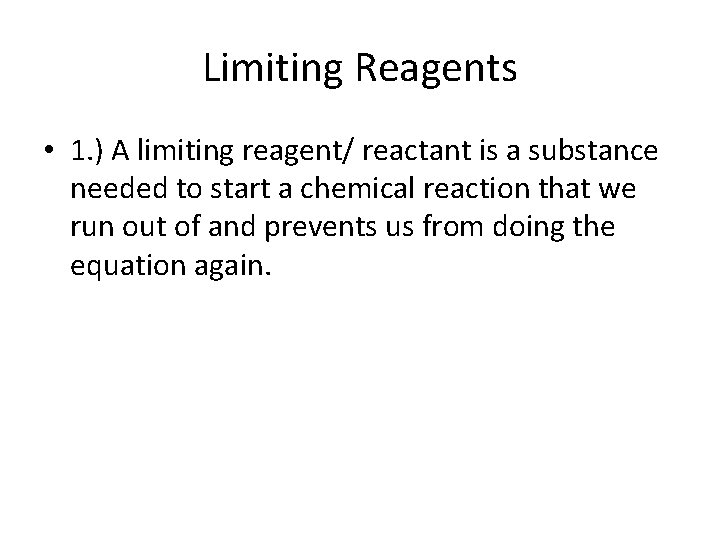
Limiting Reagents • 1. ) A limiting reagent/ reactant is a substance needed to start a chemical reaction that we run out of and prevents us from doing the equation again.

Limiting Reagents Baking or cooking recipes are good examples of limiting reactants because eventually you will run out of one of the ingredients you need Example: Let’s say you want to bake a batch of cookies. First , you google a sugar cookie recipe and find the recipe with the following ingredients (3 eggs, ½ cup milk, 1 cup flour, 2 cups sugar). Second, you find the needed ingredients and determine how many batches you can make given the ingredient amounts below.

Limiting Reagents 2. ) Which ingredient will you run out of first? 3. ) Which ingredients on hand were in excess for the recipe? Recipe Ingredients You Have 3 eggs 12 eggs ½ cup milk 2 cups milk 1 cup flour 4 cups flour 2 cups sugar 6 cups sugar

Limiting Reagents 4. ) You have 100 bolts, 150 nuts and 150 washers. You assemble a nut/bolt/washer set using the following recipe or equation. 2 washers + 1 bolt + 1 nut = 1 set • How many sets can you assemble from your supplies? • Which is the limiting component?

Limiting Reagents • We can use this same logic used above in our hotdog, cookie and nuts/bolts/washer example and apply it to chemical reactions. When dealing with a chemical equation, the chemical of which there are fewer moles than the reaction requires is the limiting reagent. In order to solve limiting reagent problems we need to follow 3 main steps.

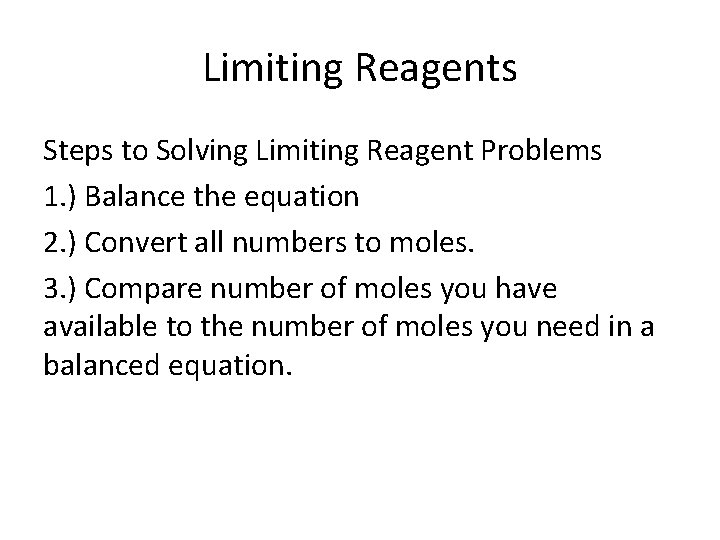
Limiting Reagents Steps to Solving Limiting Reagent Problems 1. ) Balance the equation 2. ) Convert all numbers to moles. 3. ) Compare number of moles you have available to the number of moles you need in a balanced equation.

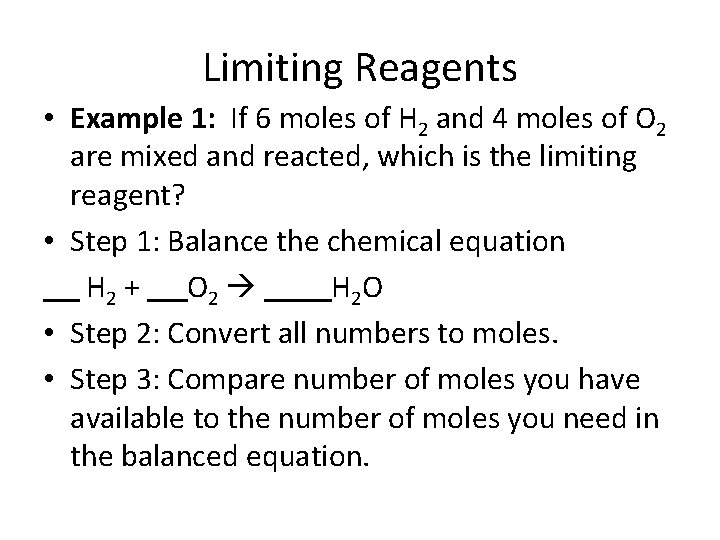
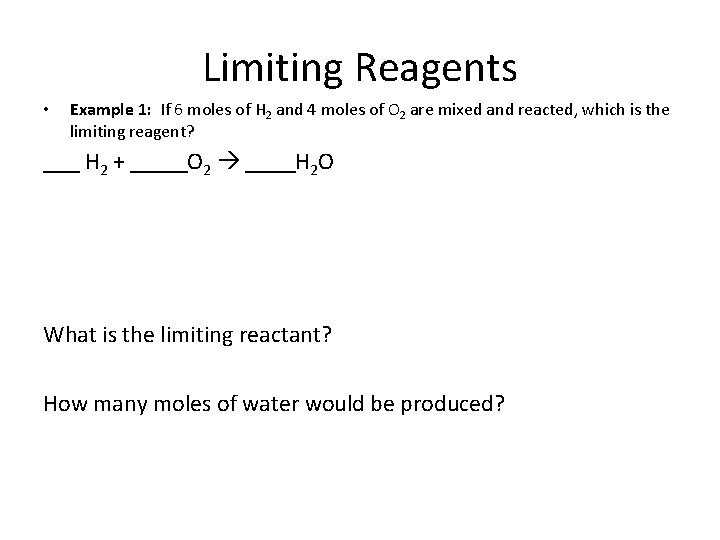
Limiting Reagents • Example 1: If 6 moles of H 2 and 4 moles of O 2 are mixed and reacted, which is the limiting reagent? • Step 1: Balance the chemical equation H 2 + O 2 H 2 O • Step 2: Convert all numbers to moles. • Step 3: Compare number of moles you have available to the number of moles you need in the balanced equation.

Limiting Reagents • Example 1: If 6 moles of H 2 and 4 moles of O 2 are mixed and reacted, which is the limiting reagent? H 2 + O 2 H 2 O What is the limiting reactant? How many moles of water would be produced?

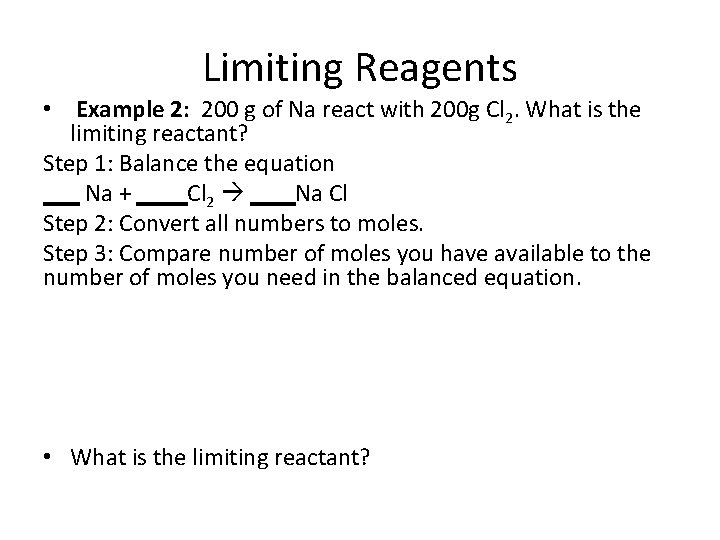
Limiting Reagents • Example 2: 200 g of Na react with 200 g Cl 2. What is the limiting reactant? Step 1: Balance the equation Na + Cl 2 Na Cl Step 2: Convert all numbers to moles. Step 3: Compare number of moles you have available to the number of moles you need in the balanced equation. • What is the limiting reactant?
Limiting Reagents • Example 3: If you had 17. 3 g of Hydrogen and 8. 91 g of oxygen, which is the limiting reagent, and how many grams of water could you produce?
Limiting Reagents • Example 4: Solid sodium and iron (III) oxide are involved in a reaction that is one of many reactions responsible for inflating a car airbag. If 100. 0 g Na and 100. 0 g Fe 2 O 3 are used, determine: 6 Na + Fe 2 O 3 3 Na 2 O + 2 Fe a. ) Limiting reactant b. ) Excess reactant c. ) Mass of solid iron produced d. ) Mass of excess reactant left over

Limiting Reagents • In order to solve this problem we will need to: a. ) Start with one reactant and turn it into the other reactant (either one is fine to start with). Compare the answer with the given amount in the problem to determine if you have more of less of what is given. From this information, the limiting reactants can be determined. b. ) Using information in A, determine the excess reactant. c. ) Determine how many grams of the product, Iron, can be formed, starting with the limiting reactant. d. ) Determine how much of your excess reactant is left over.

Limiting Reagents • Example 4: Solid sodium and iron (III) oxide are involved in a reaction that is one of many reactions responsible for inflating a car airbag. If 100. 0 g Na and 100. 0 g Fe 2 O 3 are used, determine: 6 Na + Fe 2 O 3 3 Na 2 O + 2 Fe