Single Displacement Reactions Single Displacement Reactions At the





- Slides: 15

Single Displacement Reactions

Single Displacement Reactions: At the conclusion of our time together, you should be able to: 1. Identify a basic single displacement reaction 2. Use the reactivity series table to determine if the reaction will take place 3. Balance a single displacement reaction

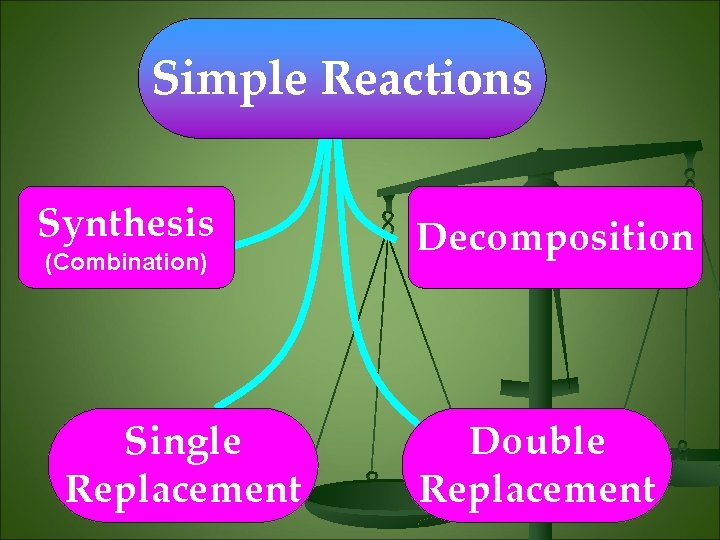
Simple Reactions Synthesis (Combination) Single Replacement Decomposition Double Replacement

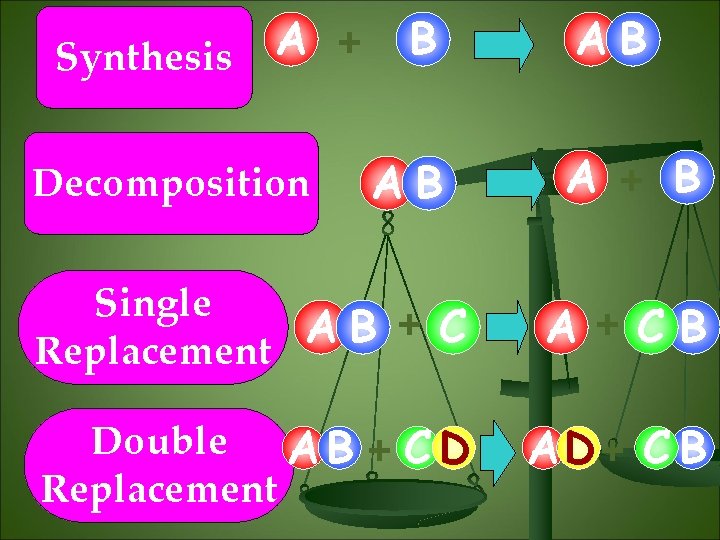
A + Synthesis Decomposition B AB AB A + B Single +C A B Replacement A +CB Double A B + C D Replacement AD + C B

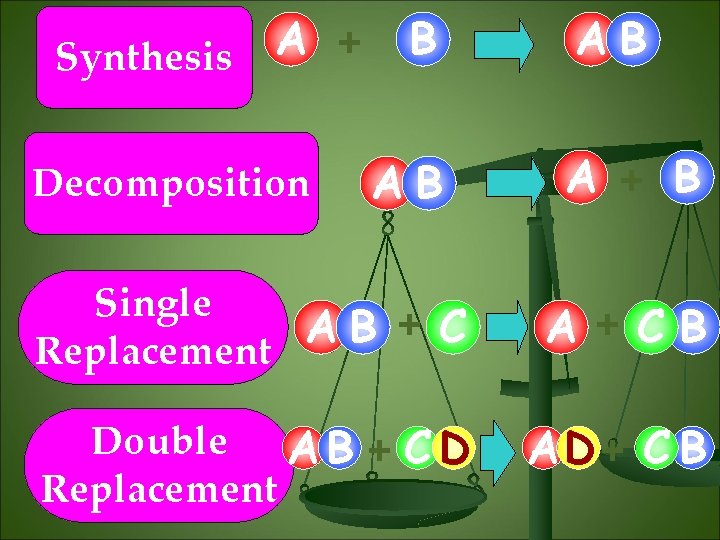
A + Synthesis Decomposition B AB AB A + B Single +C A B Replacement A +CB Double A B + C D Replacement AD + C B

3. Single Replacement Reactions • • • Single Replacement Reactions occur when one element replaces another in a compound. A metal can replace a metal (+) OR a halogen can replace a halogen (-). element + compound A + BC AC + B (if A is a metal) OR A + BC BA + C (if A is a halogen ) (remember the cation always goes first!)

3. Single Replacement Reactions • For these reactions, you must remember: When H 2 O splits into ions, it splits into H+ and OH- (not H+ and O-2 !!) A single replacement reaction will only occur if the replacement ion has a higher activity than the original ion in the compound To determine this, refer to a relative activity table

Relative Activities Metals Decreasing Activity Lithium, Li Potassium, K Calcium, Ca Sodium, Na Magnesium, Mg Aluminum, Al Zinc, Zn Hydrogen, H Chromium, Cr Copper, Cu Iron, Fe Mercury, Hg Nickel, Ni Silver, Ag Tin, Sn Platinum, Pt Lead, Pb Gold, Au Halogens Fluorine, F Chlorine, Cl Bromine, Br Iodine, I

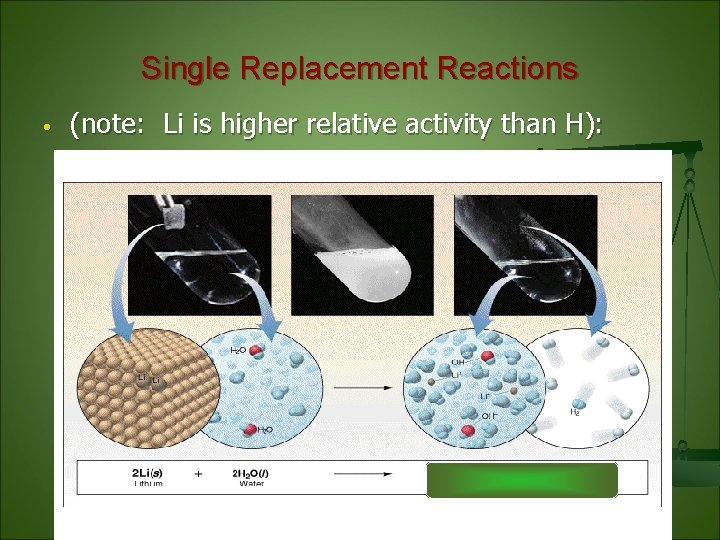
Single Replacement Reactions • (note: Li is higher relative activity than H):

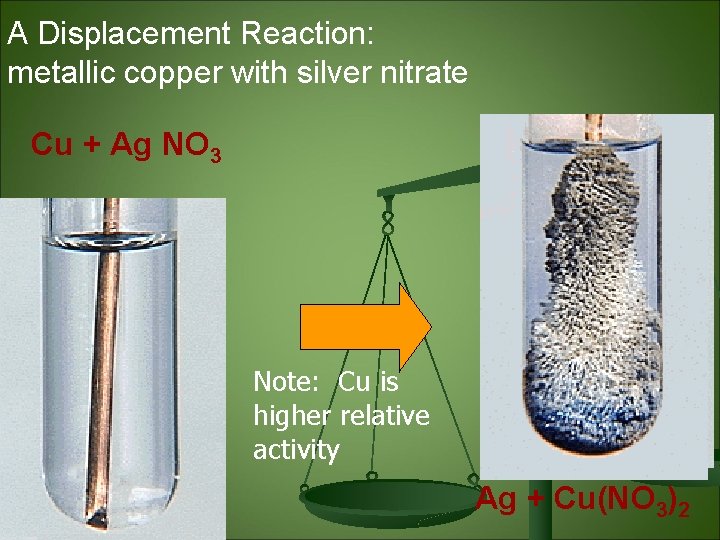
A Displacement Reaction: metallic copper with silver nitrate Cu + Ag NO 3 Note: Cu is higher relative activity Ag + Cu(NO 3)2

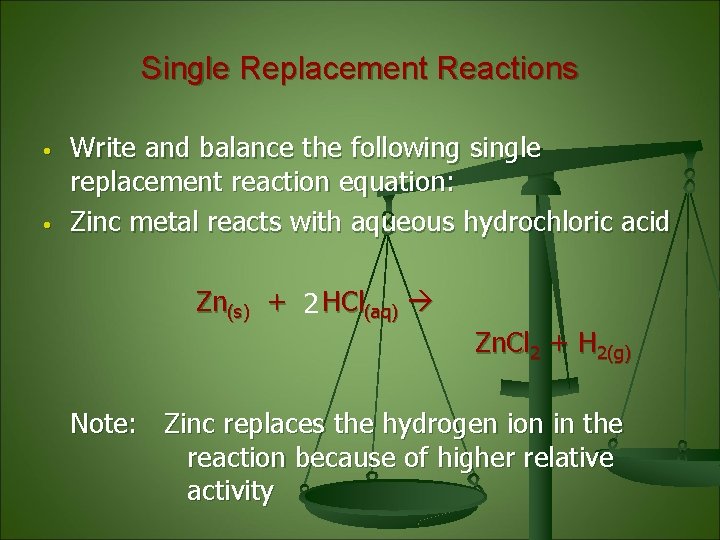
Single Replacement Reactions • • Write and balance the following single replacement reaction equation: Zinc metal reacts with aqueous hydrochloric acid Zn(s) + 2 HCl(aq) Zn. Cl 2 + H 2(g) Note: Zinc replaces the hydrogen ion in the reaction because of higher relative activity

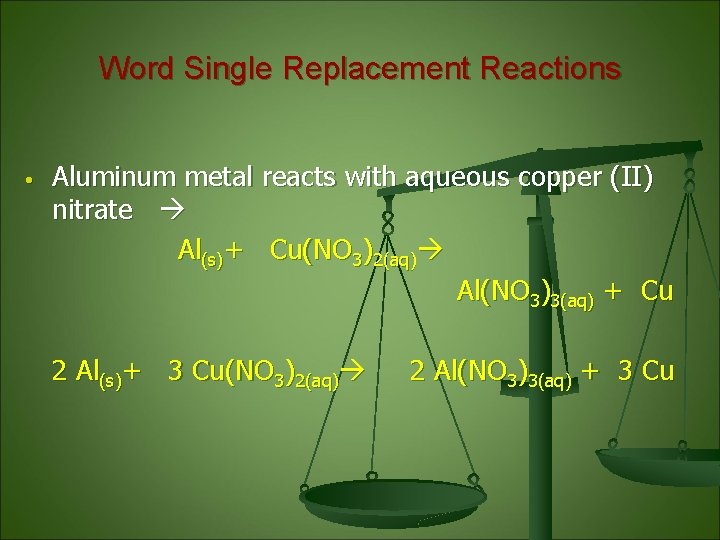
Word Single Replacement Reactions • Aluminum metal reacts with aqueous copper (II) nitrate Al(s)+ Cu(NO 3)2(aq) Al(NO 3)3(aq) + Cu 2 Al(s)+ 3 Cu(NO 3)2(aq) 2 Al(NO 3)3(aq) + 3 Cu

Single Displacement Reactions: Let’s see if you can: 1. Identify a basic single displacement reaction 2. Use the reactivity series table to determine if the reaction will take place 3. Balance a single displacement reaction

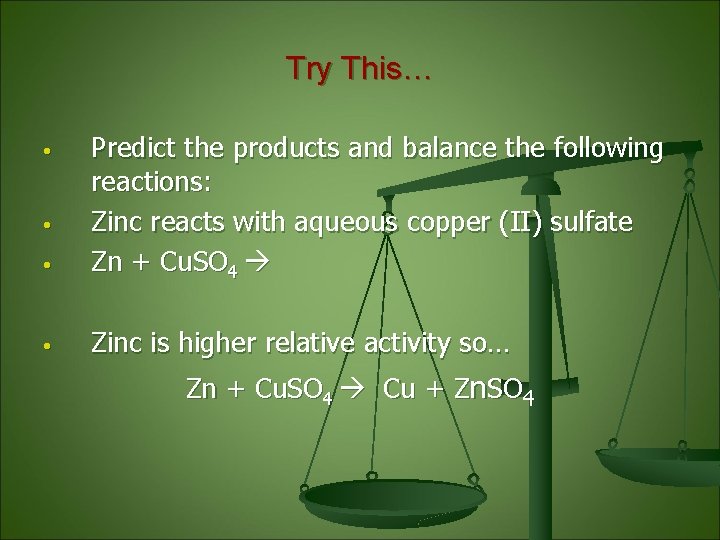
Try This… • Predict the products and balance the following reactions: Zinc reacts with aqueous copper (II) sulfate Zn + Cu. SO 4 • Zinc is higher relative activity so… • • Zn + Cu. SO 4 Cu + Zn. SO 4

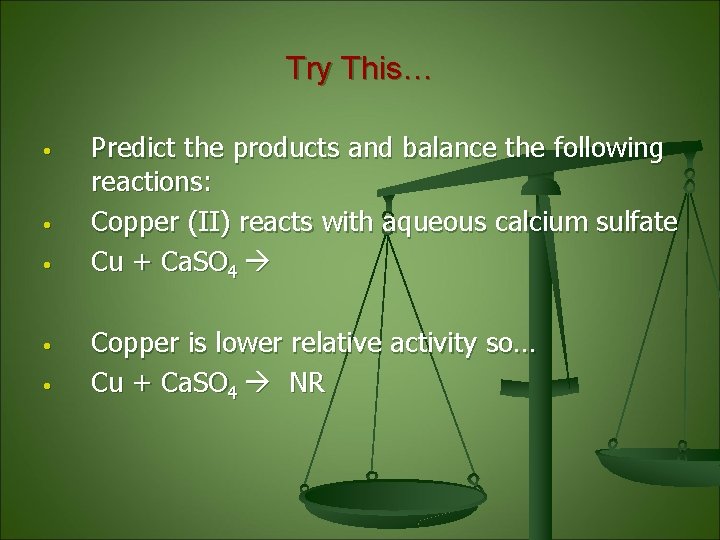
Try This… • • • Predict the products and balance the following reactions: Copper (II) reacts with aqueous calcium sulfate Cu + Ca. SO 4 Copper is lower relative activity so… Cu + Ca. SO 4 NR
 Single displacement vs double displacement
Single displacement vs double displacement Reactivity series and displacement reactions
Reactivity series and displacement reactions Redox reactions examples
Redox reactions examples Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Types of reactions
Types of reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Predicting single replacement reactions
Predicting single replacement reactions General equation for single displacement reaction
General equation for single displacement reaction Single displacement
Single displacement The equation a + bx ax + b is the general equation for a
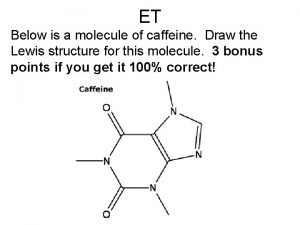
The equation a + bx ax + b is the general equation for a Draw the structure of caffeine
Draw the structure of caffeine Single replacement reaction cartoon
Single replacement reaction cartoon Predicting single replacement reactions
Predicting single replacement reactions Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau