1 calcium hydrochloric acid HCl a Ca HCl




- Slides: 26


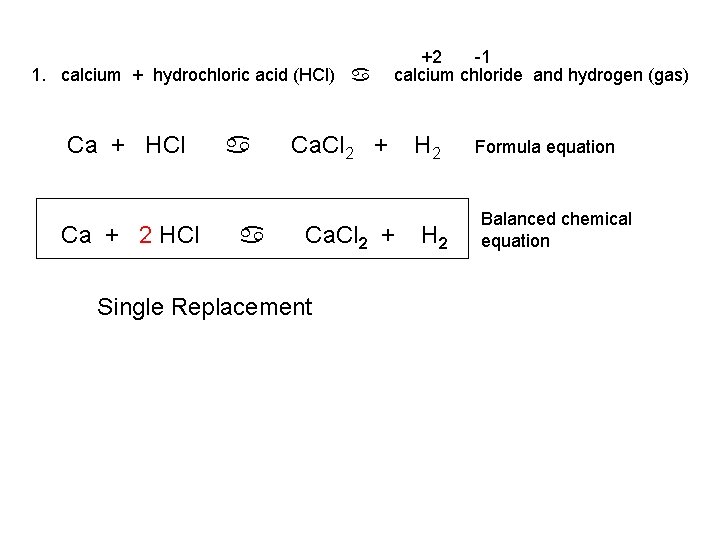
1. calcium + hydrochloric acid (HCl) a Ca + HCl Ca + 2 HCl a a +2 -1 calcium chloride and hydrogen (gas) Ca. Cl 2 + Single Replacement H 2 Formula equation Balanced chemical equation

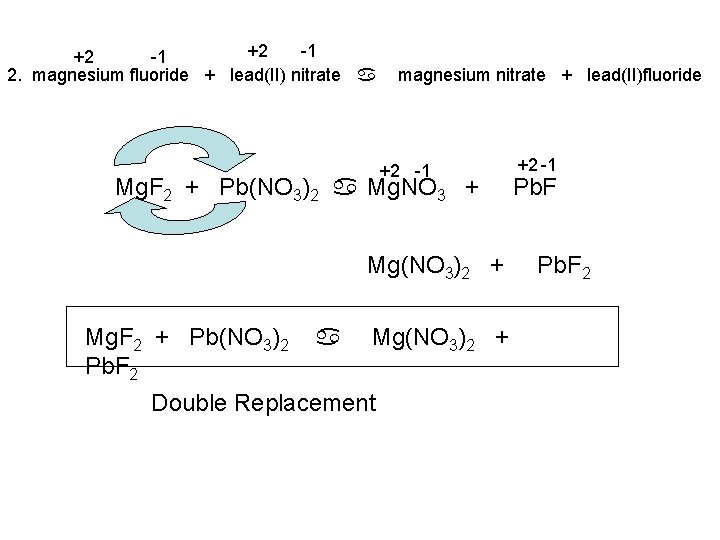
+2 -1 2. magnesium fluoride + lead(II) nitrate a magnesium nitrate + lead(II)fluoride +2 -1 Mg. F 2 + Pb(NO 3)2 a Mg. NO 3 + Mg(NO 3)2 + Mg. F 2 + Pb(NO 3)2 Pb. F 2 a Mg(NO 3)2 + Double Replacement +2 -1 Pb. F 2

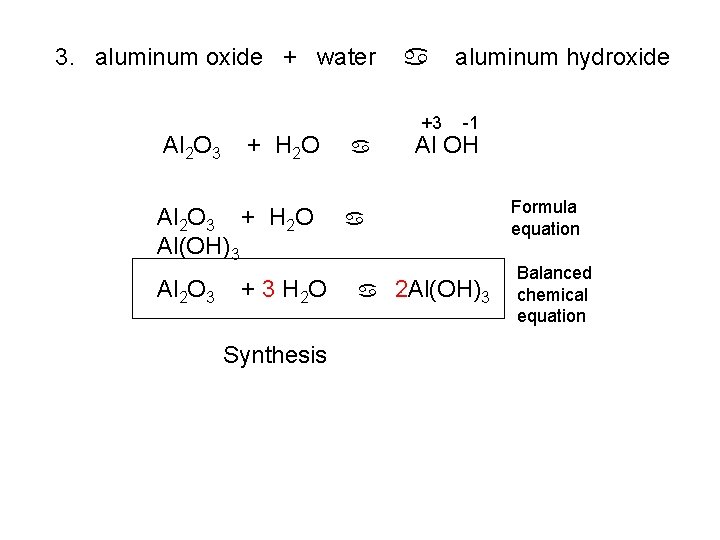
3. aluminum oxide + water +3 -2 Al O + H 2 O a a aluminum hydroxide

3. aluminum oxide + water Al 2 O 3 + H 2 O Al(OH)3 Al 2 O 3 + 3 H 2 O Synthesis a +3 a aluminum hydroxide -1 Al OH Formula equation a a 2 Al(OH)3 Balanced chemical equation

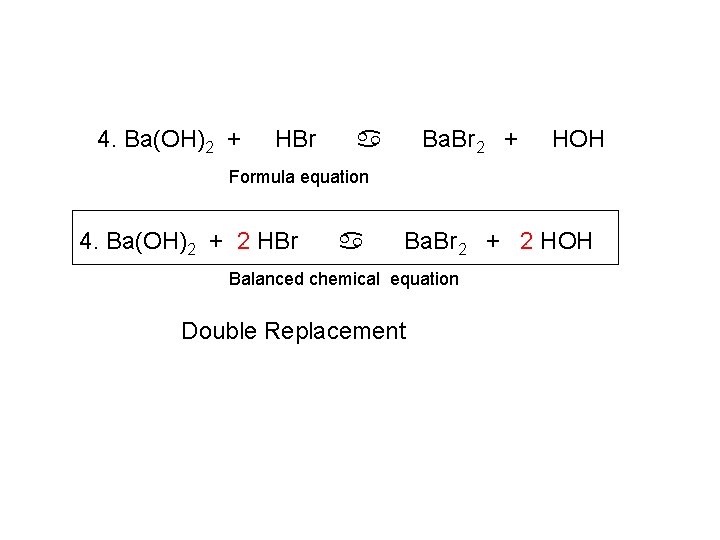
4. Ba(OH)2 + HBr Ba. Br 2 + a HOH Formula equation 4. Ba(OH)2 + 2 HBr a Ba. Br 2 + 2 HOH Balanced chemical equation Double Replacement

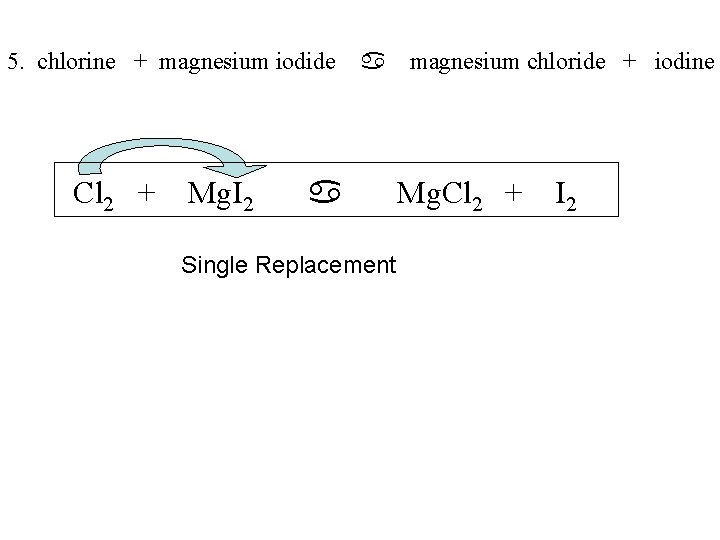
5. chlorine + magnesium iodide Cl 2 + Mg. I 2 a a Single Replacement magnesium chloride + iodine Mg. Cl 2 + I 2

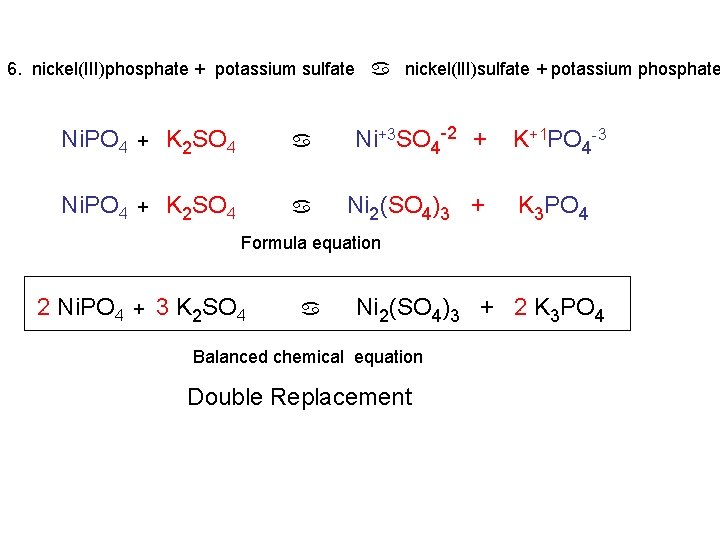
6. nickel(III)phosphate + potassium sulfate a nickel(III)sulfate + potassium phosphate Ni+3 PO 4 -3 + K+1 SO 4 -2 a

6. nickel(III)phosphate + potassium sulfate a nickel(III)sulfate + potassium phosphate Ni. PO 4 + K 2 SO 4 a Ni+3 SO 4 -2 + K+1 PO 4 -3 Ni. PO 4 + K 2 SO 4 a Ni 2(SO 4)3 + K 3 PO 4 Formula equation 2 Ni. PO 4 + 3 K 2 SO 4 a Ni 2(SO 4)3 + 2 K 3 PO 4 Balanced chemical equation Double Replacement

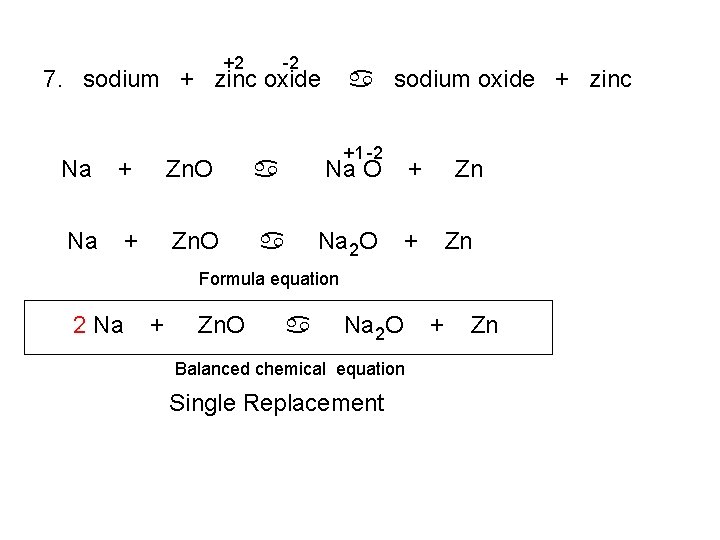
+2 -2 7. sodium + zinc oxide a sodium oxide + zinc +1 -2 Na + Zn. O a Na O + Na + Zn. O a Na 2 O + Zn Zn Formula equation 2 Na + Zn. O a Na 2 O Balanced chemical equation Single Replacement + Zn

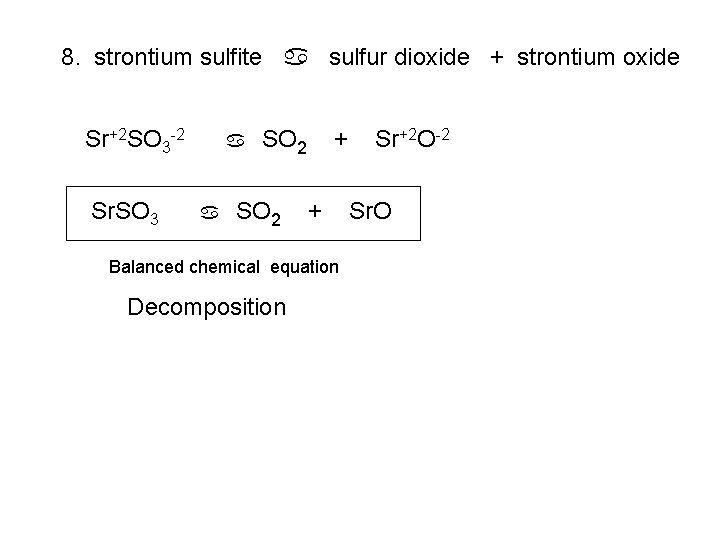
8. strontium sulfite a sulfur dioxide + strontium oxide Sr+2 SO 3 -2 Sr. SO 3 a a SO 2 + + Balanced chemical equation Decomposition Sr+2 O-2 Sr. O

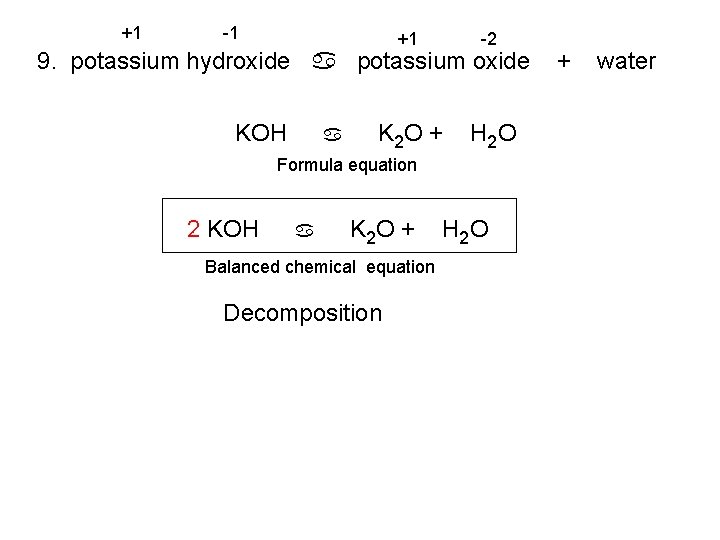
+1 -1 +1 -2 K 2 O + H 2 O 9. potassium hydroxide a potassium oxide KOH a Formula equation 2 KOH a K 2 O + Balanced chemical equation Decomposition H 2 O + water

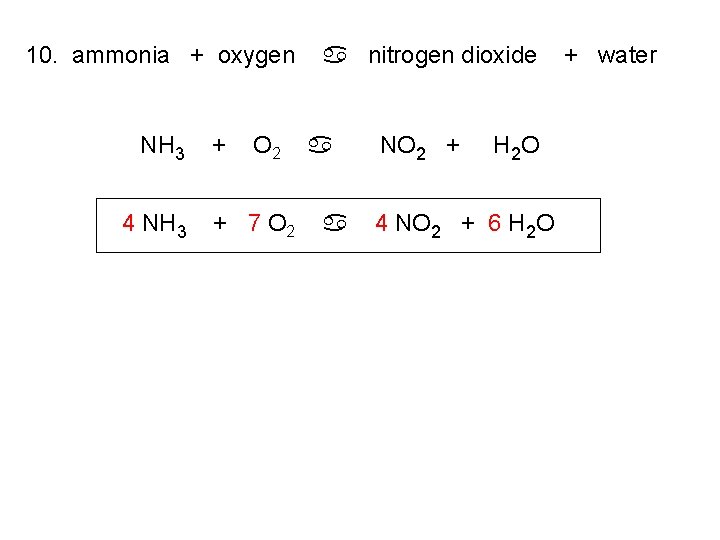
10. ammonia + oxygen NH 3 4 NH 3 + O 2 + 7 O 2 a nitrogen dioxide a a NO 2 + H 2 O 4 NO 2 + 6 H 2 O + water

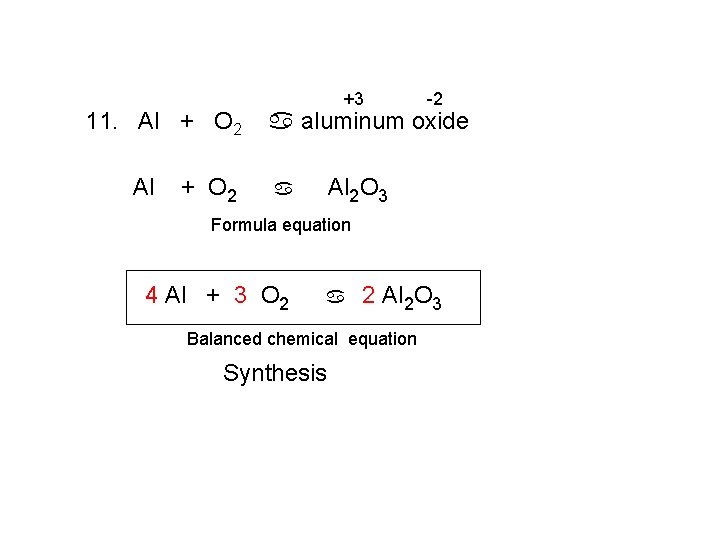
11. Al + O 2 +3 -2 a aluminum oxide Al 2 O 3 a Formula equation 4 Al + 3 O 2 a 2 Al 2 O 3 Balanced chemical equation Synthesis

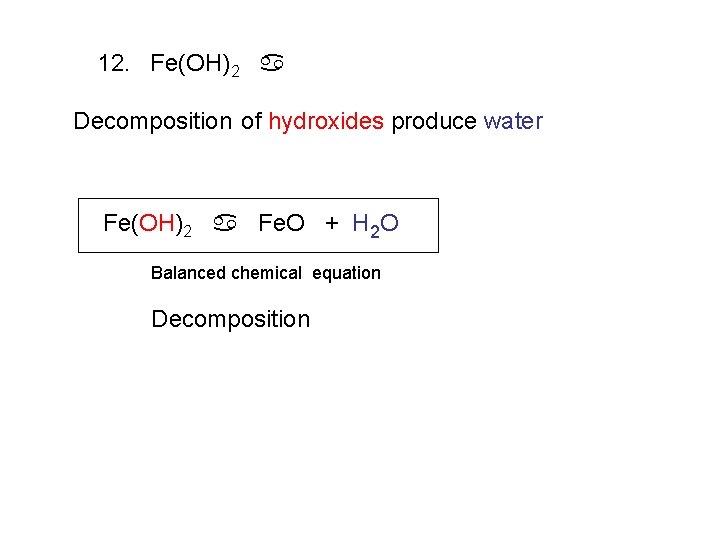
12. Fe(OH)2 a Decomposition of hydroxides produce water Fe(OH)2 a Fe. O + H 2 O Balanced chemical equation Decomposition

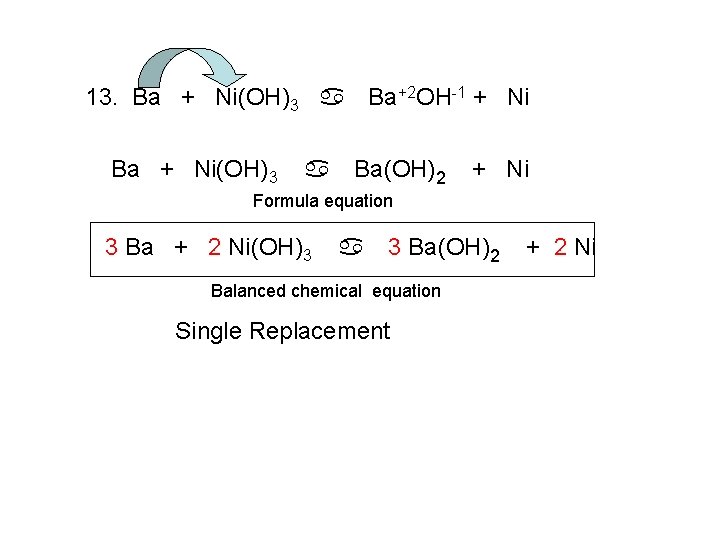
13. Ba + Ni(OH)3 a Ba+2 OH-1 + Ni Ba + Ni(OH)3 a Ba(OH)2 + Ni Formula equation 3 Ba + 2 Ni(OH)3 a 3 Ba(OH)2 Balanced chemical equation Single Replacement + 2 Ni

14. sodium carbonate a Decomposition of carbonates produce CO 2 Na 2 CO 3 a Na 2 O + CO 2 Balanced chemical equation Decomposition

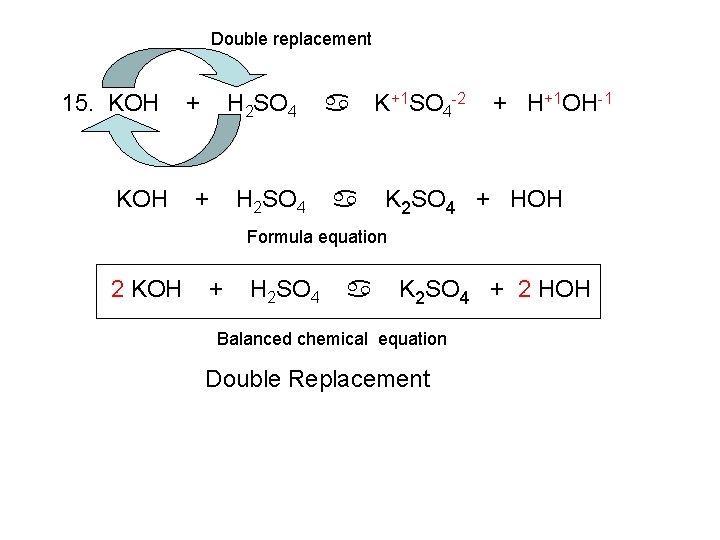
Double replacement 15. KOH + H 2 SO 4 a a K+1 SO 4 -2 + H+1 OH-1 K 2 SO 4 + HOH Formula equation 2 KOH + H 2 SO 4 a K 2 SO 4 + 2 HOH Balanced chemical equation Double Replacement

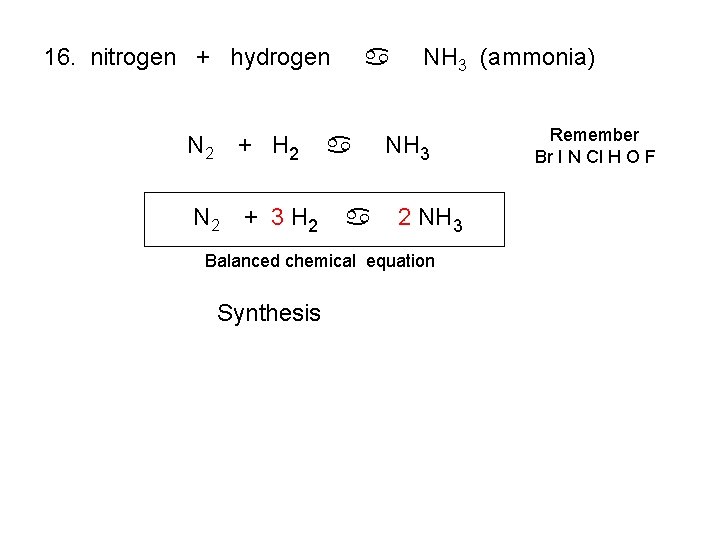
16. nitrogen + hydrogen N 2 + H 2 N 2 + 3 H 2 a a a NH 3 (ammonia) NH 3 2 NH 3 Balanced chemical equation Synthesis Remember Br I N Cl H O F

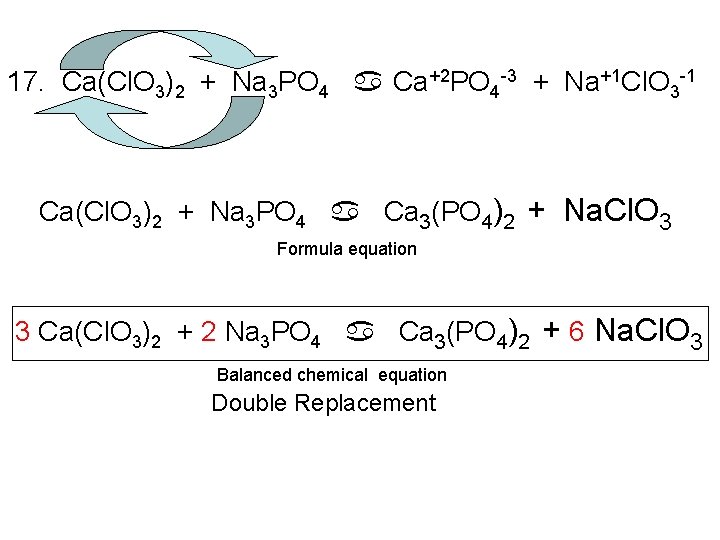
17. Ca(Cl. O 3)2 + Na 3 PO 4 a Ca+2 PO 4 -3 + Na+1 Cl. O 3 -1 Ca(Cl. O 3)2 + Na 3 PO 4 a Ca 3(PO 4)2 + Na. Cl. O 3 Formula equation 3 Ca(Cl. O 3)2 + 2 Na 3 PO 4 a Ca 3(PO 4)2 + 6 Na. Cl. O 3 Balanced chemical equation Double Replacement

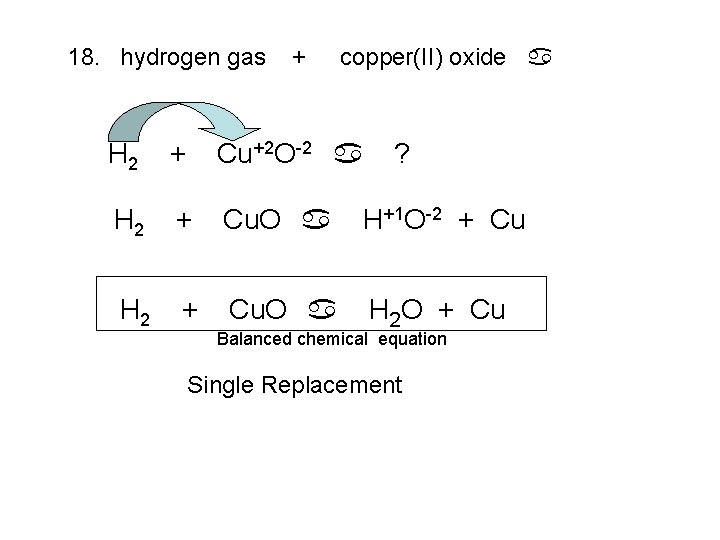
18. hydrogen gas + copper(II) oxide a H 2 + Cu+2 O-2 a ? H 2 + Cu. O a H+1 O-2 + Cu H 2 + Cu. O a H 2 O + Cu Balanced chemical equation Single Replacement

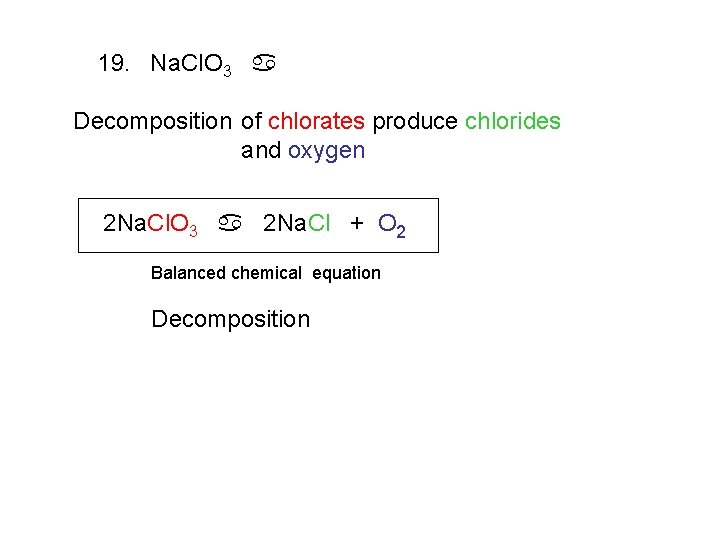
19. Na. Cl. O 3 a Decomposition of chlorates produce chlorides and oxygen 2 Na. Cl. O 3 a 2 Na. Cl + O 2 Balanced chemical equation Decomposition

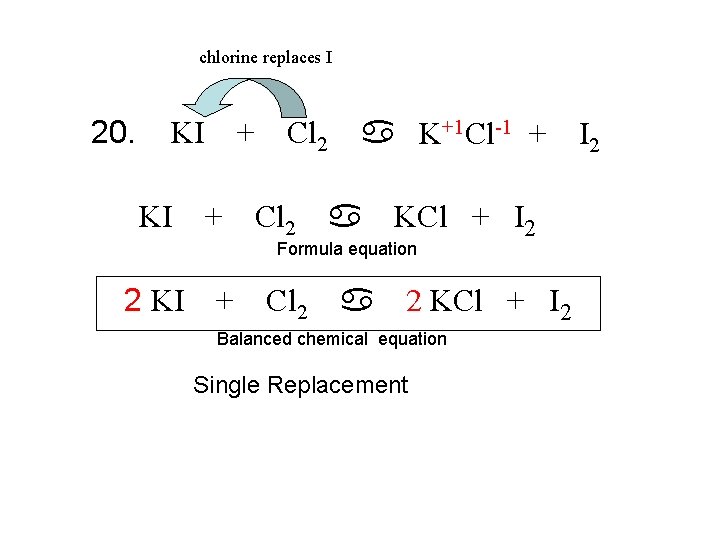
chlorine replaces I 20. + Cl 2 a K+1 Cl-1 + KI KI + Cl 2 a KCl + I 2 Formula equation 2 KI + Cl 2 a 2 KCl + I 2 Balanced chemical equation Single Replacement I 2

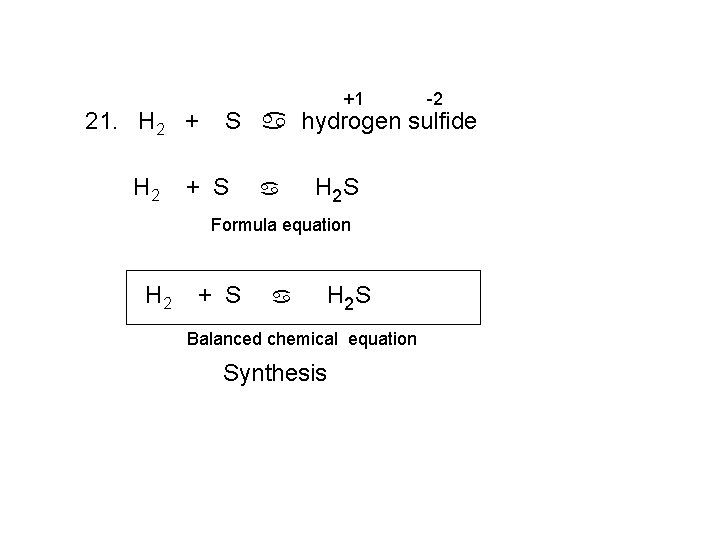
21. H 2 +1 S a hydrogen sulfide + S a H 2 S Formula equation H 2 -2 + S a H 2 S Balanced chemical equation Synthesis

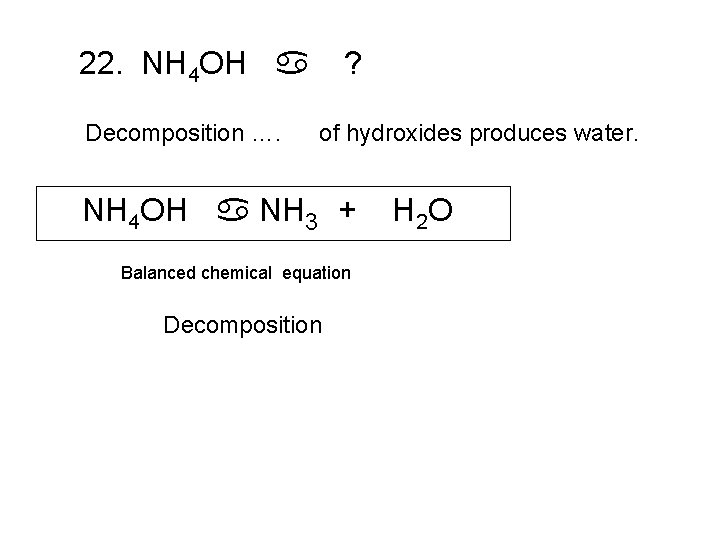
22. NH 4 OH a Decomposition …. ? of hydroxides produces water. NH 4 OH a NH ? 3 + Balanced chemical equation Decomposition H 2 O

0– 4 A 5– 8 B 9 – 12 C 13 – 16 D