Stoichiometry Predicting amounts of reagents needed or amounts

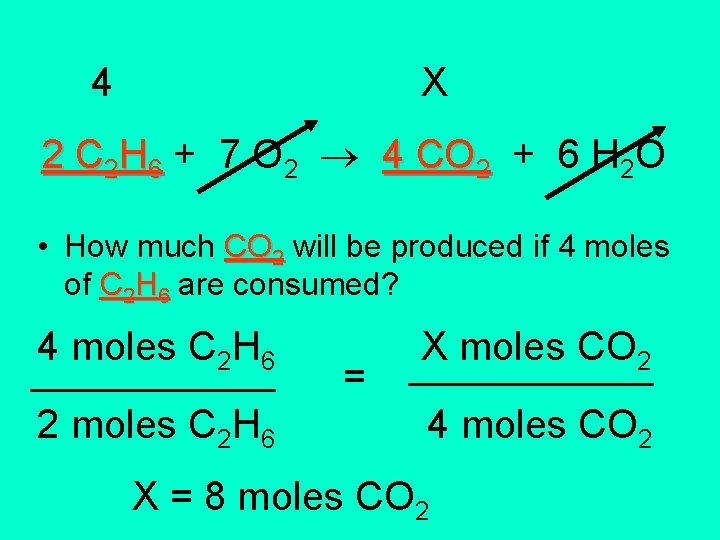
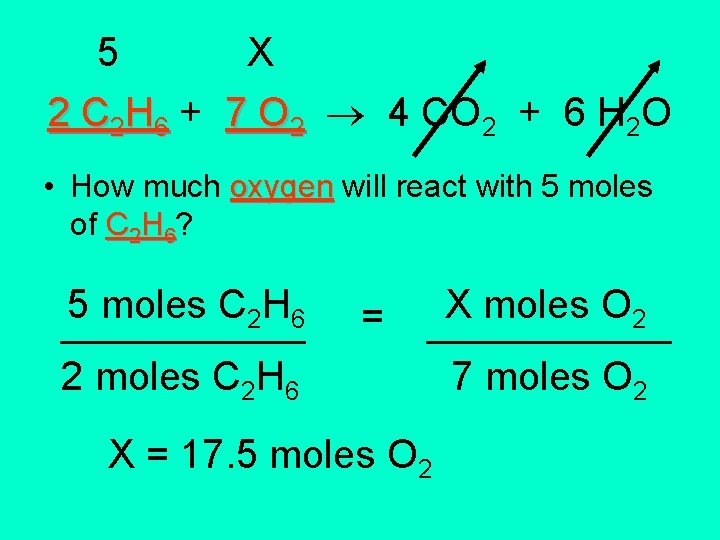
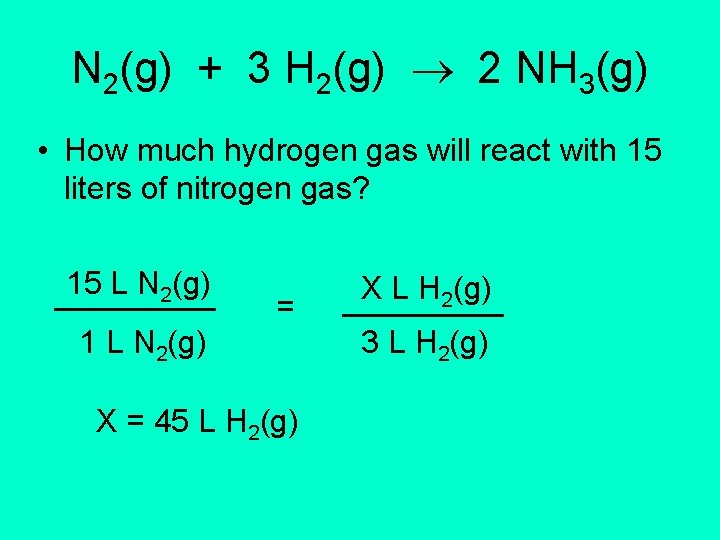
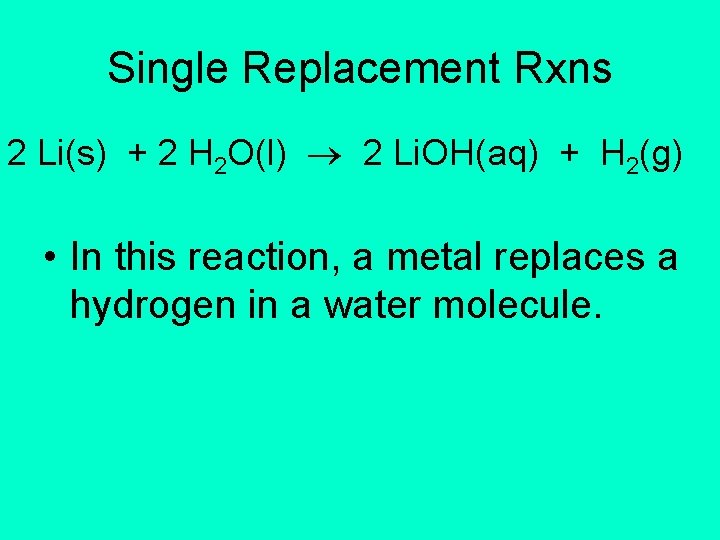
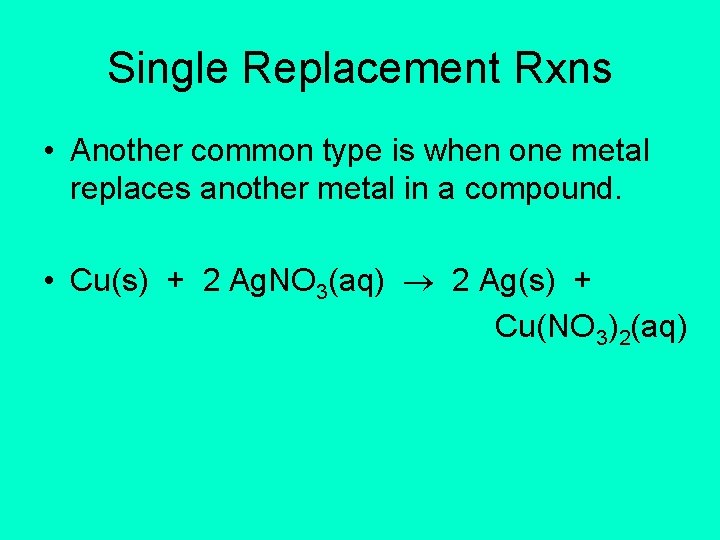








- Slides: 35

Stoichiometry Predicting amounts of reagents needed or amounts of products made

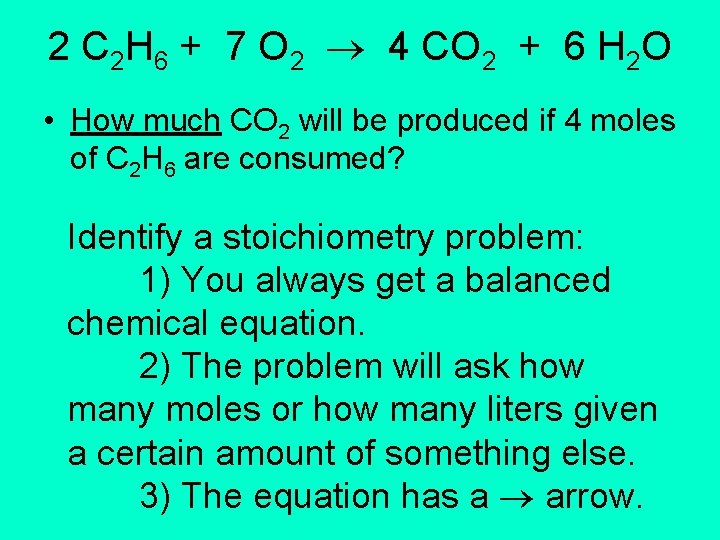
2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O • How much CO 2 will be produced if 4 moles of C 2 H 6 are consumed? Identify a stoichiometry problem: 1) You always get a balanced chemical equation. 2) The problem will ask how many moles or how many liters given a certain amount of something else. 3) The equation has a arrow.
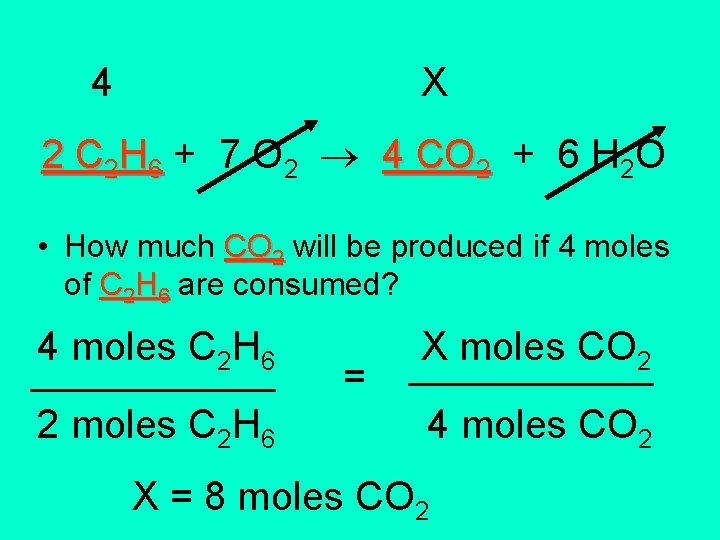
4 X 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O • How much CO 2 will be produced if 4 moles of C 2 H 6 are consumed? 4 moles C 2 H 6 ______ 2 moles C 2 H 6 = X moles CO ______2 4 moles CO 2 X = 8 moles CO 2

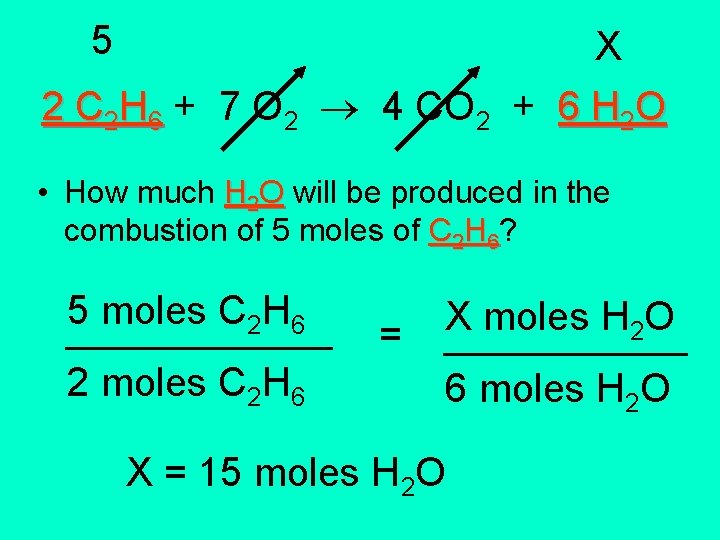
5 X 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O • How much H 2 O will be produced in the combustion of 5 moles of C 2 H 6? 5 moles C H 2 6 ______ 2 moles C 2 H 6 = X moles H O 2 ______ 6 moles H 2 O X = 15 moles H 2 O
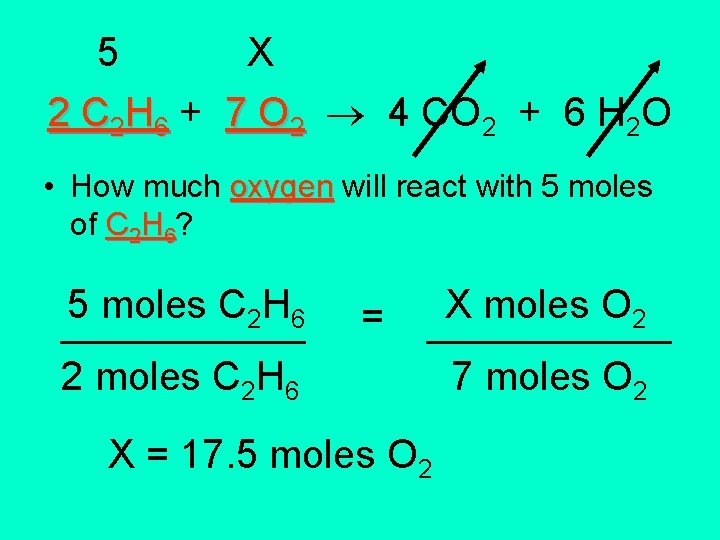
5 X 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O • How much oxygen will react with 5 moles of C 2 H 6? 5 moles C H 2 ______6 2 moles C 2 H 6 = X moles O 2 ______ 7 moles O 2 X = 17. 5 moles O 2

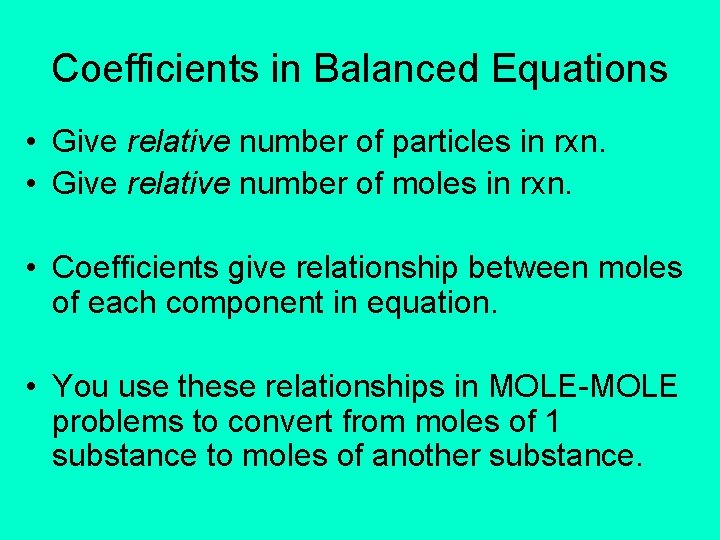
Coefficients in Balanced Equations • Give relative number of particles in rxn. • Give relative number of moles in rxn. • Coefficients give relationship between moles of each component in equation. • You use these relationships in MOLE-MOLE problems to convert from moles of 1 substance to moles of another substance.

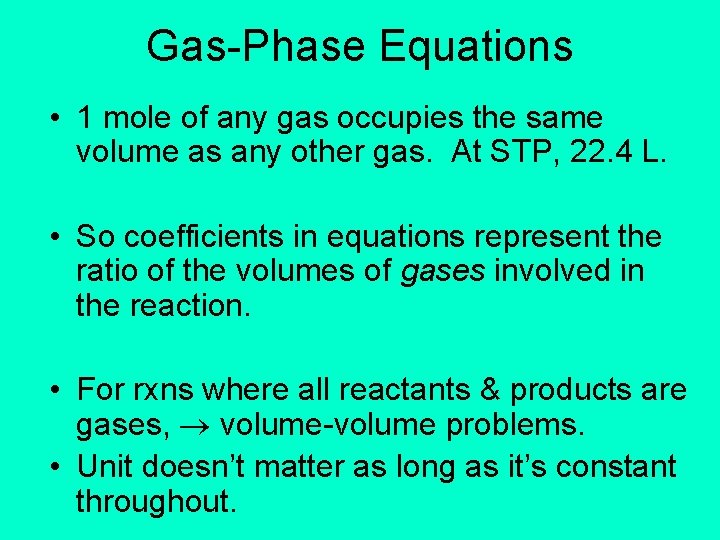
Gas-Phase Equations • 1 mole of any gas occupies the same volume as any other gas. At STP, 22. 4 L. • So coefficients in equations represent the ratio of the volumes of gases involved in the reaction. • For rxns where all reactants & products are gases, volume-volume problems. • Unit doesn’t matter as long as it’s constant throughout.
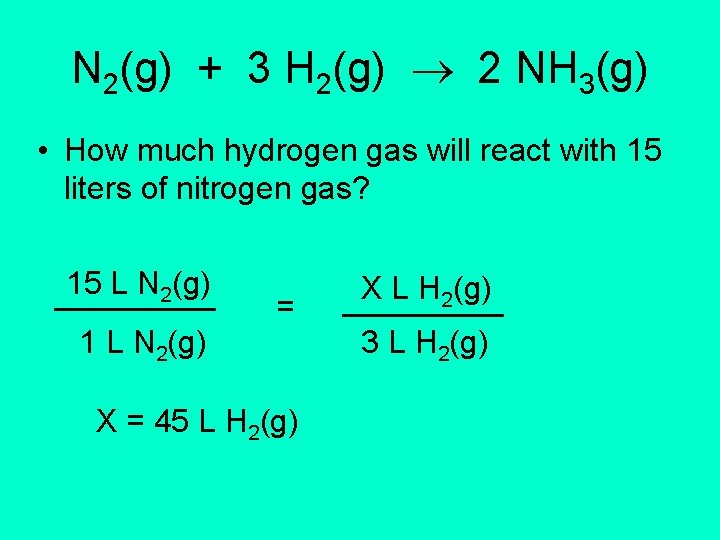
N 2(g) + 3 H 2(g) 2 NH 3(g) • How much hydrogen gas will react with 15 liters of nitrogen gas? 15 L N 2(g) _____ 1 L N 2(g) = X = 45 L H 2(g) X L H 2(g) _____ 3 L H 2(g)

A word about ….

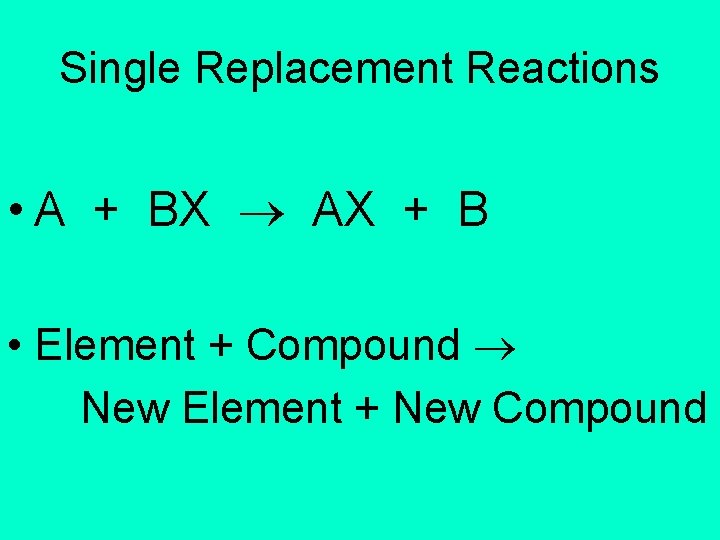
Single Replacement Reactions • A + BX AX + B • Element + Compound New Element + New Compound
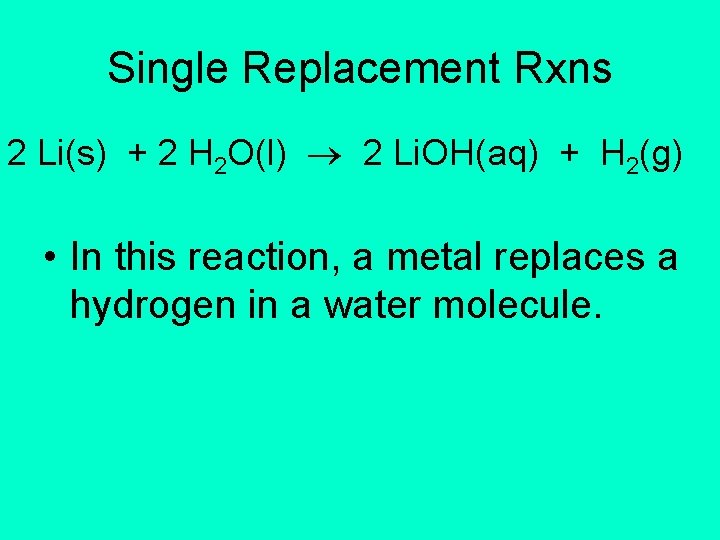
Single Replacement Rxns 2 Li(s) + 2 H 2 O(l) 2 Li. OH(aq) + H 2(g) • In this reaction, a metal replaces a hydrogen in a water molecule.
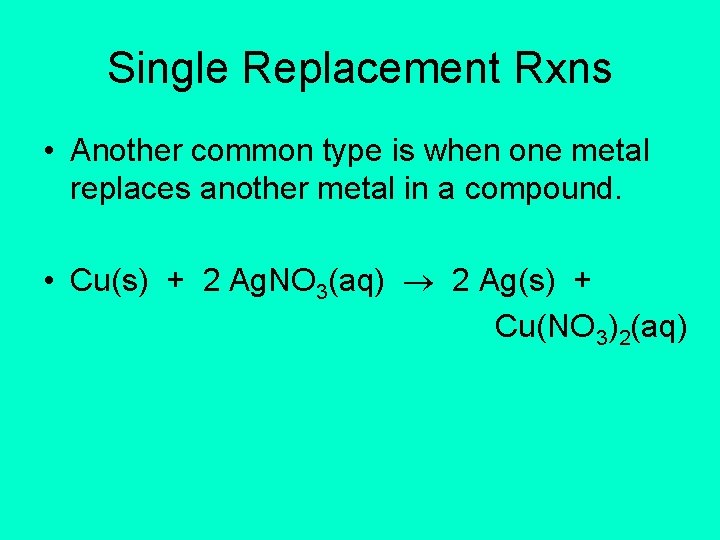
Single Replacement Rxns • Another common type is when one metal replaces another metal in a compound. • Cu(s) + 2 Ag. NO 3(aq) 2 Ag(s) + Cu(NO 3)2(aq)

Single Replacement Reactions • Metals have different reactivities. • You have to predict if a given metal and a given compound will react or not.

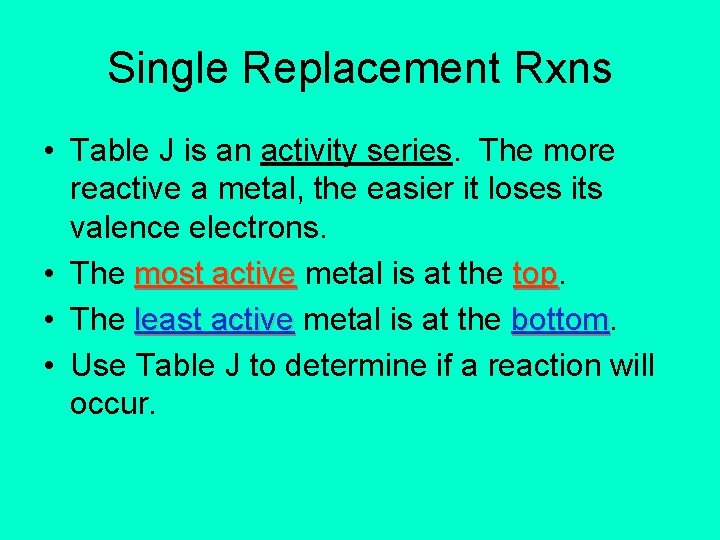
Single Replacement Rxns • Table J is an activity series. The more reactive a metal, the easier it loses its valence electrons. • The most active metal is at the top • The least active metal is at the bottom • Use Table J to determine if a reaction will occur.

Single Replacement Rxns • The rule is: • A metal can replace any metal listed below it that is in a compound. • It cannot replace any metal listed above it.

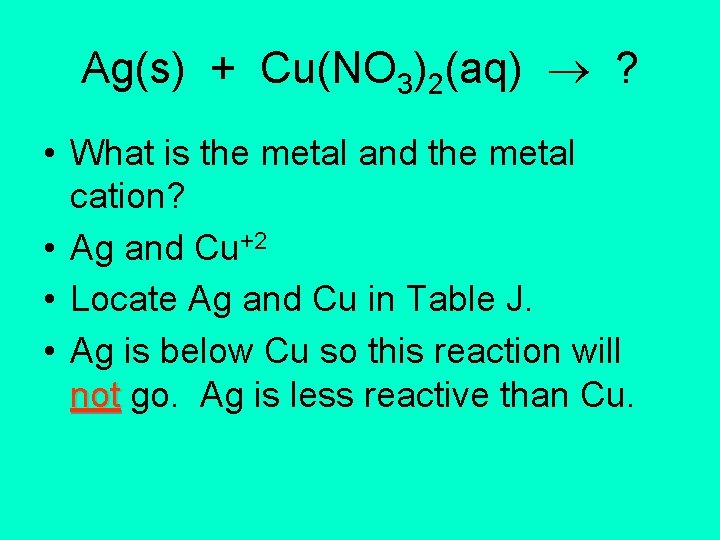
Ag(s) + Cu(NO 3)2(aq) ? • What is the metal and the metal cation? • Ag and Cu+2 • Locate Ag and Cu in Table J. • Ag is below Cu so this reaction will not go. Ag is less reactive than Cu.

Cu(s) + 2 Ag. NO 3(aq) ? • • What is the metal and the metal cation? Cu and Ag+1 Locate Cu and Ag in Table J. Cu is above Ag in Table J. Cu is more reactive than Ag. This reaction will go. • 2 Ag(s) + 2 Cu(NO 3)2(aq)

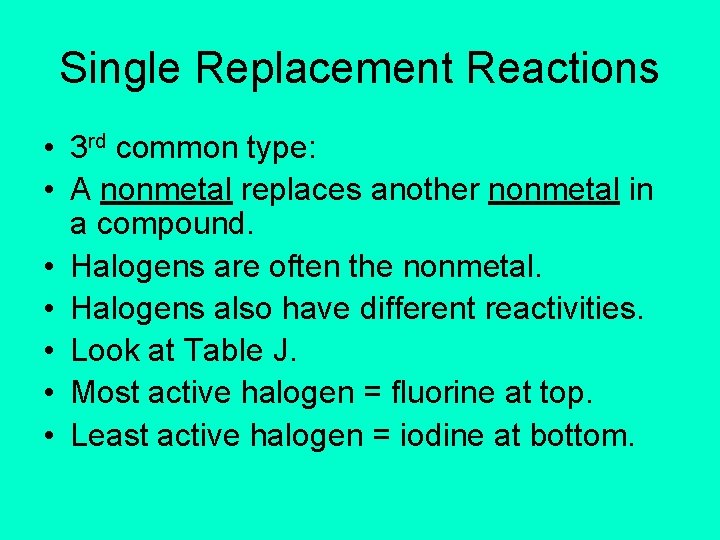
Single Replacement Reactions • 3 rd common type: • A nonmetal replaces another nonmetal in a compound. • Halogens are often the nonmetal. • Halogens also have different reactivities. • Look at Table J. • Most active halogen = fluorine at top. • Least active halogen = iodine at bottom.

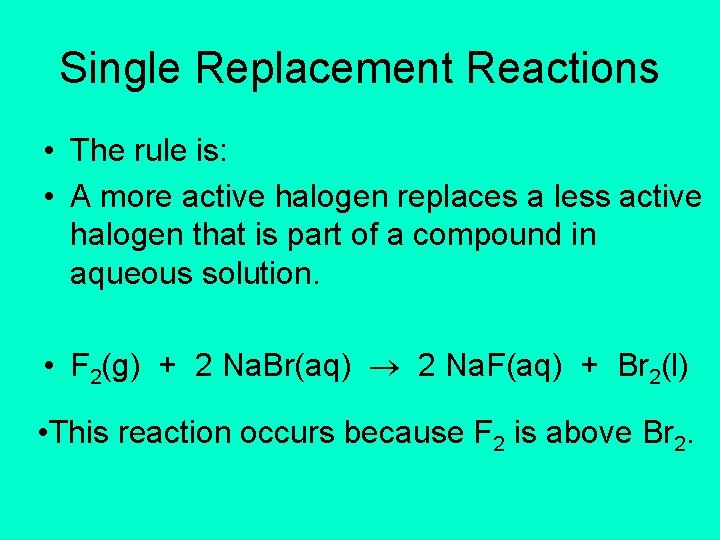
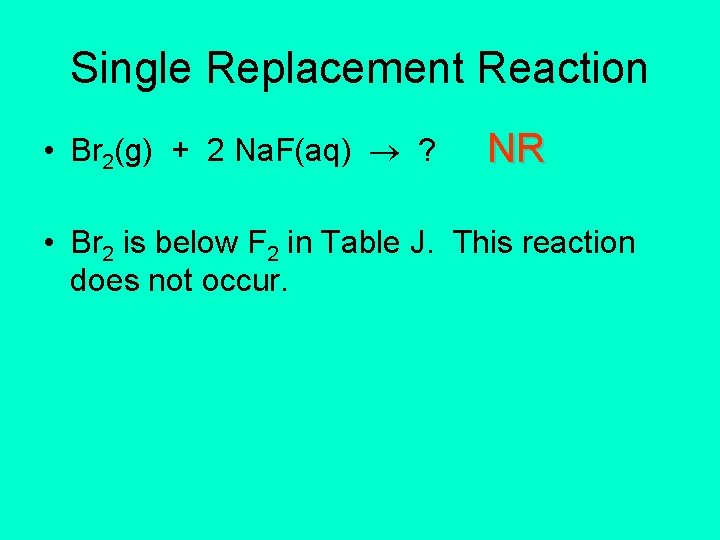
Single Replacement Reactions • The rule is: • A more active halogen replaces a less active halogen that is part of a compound in aqueous solution. • F 2(g) + 2 Na. Br(aq) 2 Na. F(aq) + Br 2(l) • This reaction occurs because F 2 is above Br 2.

Single Replacement Reaction • Br 2(g) + 2 Na. F(aq) ? NR • Br 2 is below F 2 in Table J. This reaction does not occur.

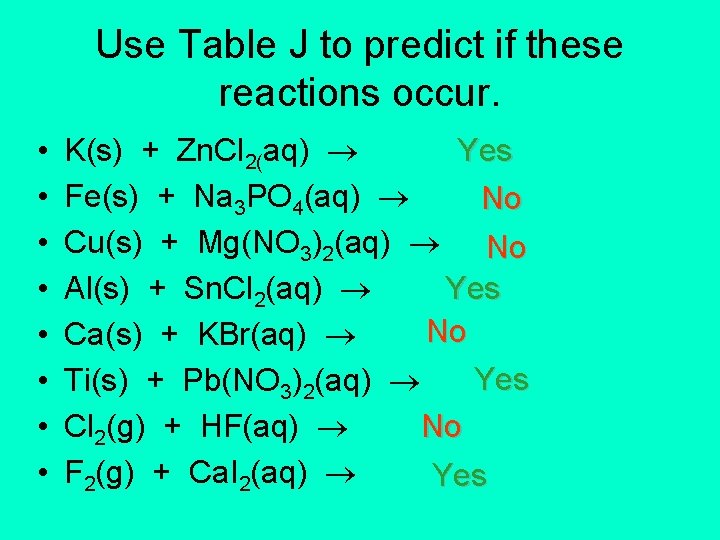
Use Table J to predict if these reactions occur. • • K(s) + Zn. Cl 2(aq) Yes Fe(s) + Na 3 PO 4(aq) No Cu(s) + Mg(NO 3)2(aq) No Yes Al(s) + Sn. Cl 2(aq) No Ca(s) + KBr(aq) Yes Ti(s) + Pb(NO 3)2(aq) No Cl 2(g) + HF(aq) F 2(g) + Ca. I 2(aq) Yes

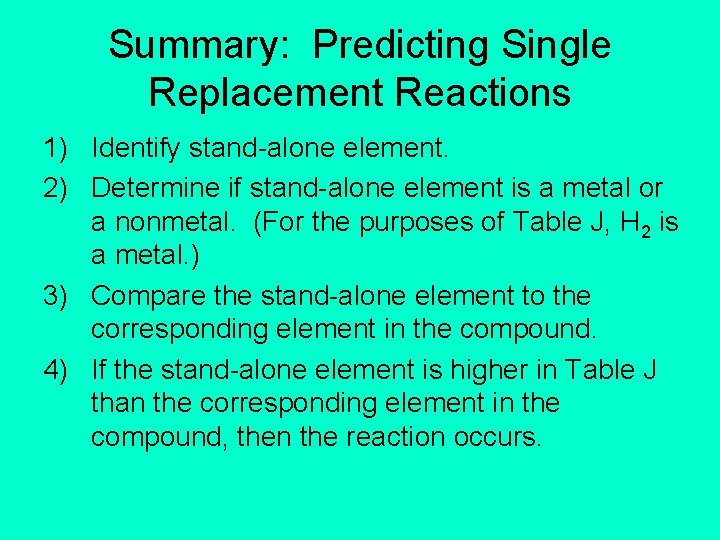
Summary: Predicting Single Replacement Reactions 1) Identify stand-alone element. 2) Determine if stand-alone element is a metal or a nonmetal. (For the purposes of Table J, H 2 is a metal. ) 3) Compare the stand-alone element to the corresponding element in the compound. 4) If the stand-alone element is higher in Table J than the corresponding element in the compound, then the reaction occurs.

A word about …

Reactions in aqueous solution • Many reactions, esp. many double replacement reactions, occur in water. • What happens when substances dissolve in water? • Depends on if they are ionic or covalent.

Dissolving • Covalent substance – sugar or C 6 H 12 O 6 • C 6 H 12 O 6(s) C 6 H 12 O 6(aq) • The sugar molecules are spread out among the water molecules.

Dissolving • Ionic substance – table salt or Na. Cl • Na. Cl(s) Na+(aq) + Cl-(aq) • The ions are spread out among the water molecules.

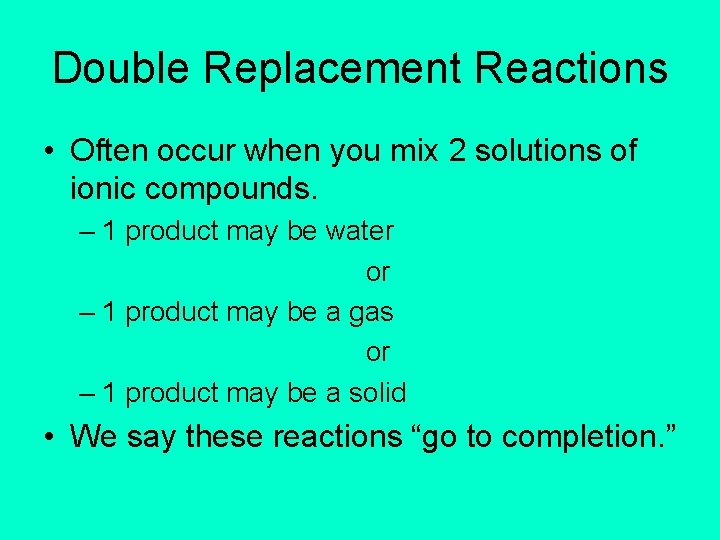
Double Replacement Reactions • Often occur when you mix 2 solutions of ionic compounds. – 1 product may be water or – 1 product may be a gas or – 1 product may be a solid • We say these reactions “go to completion. ”

Reactions producing Solids • • Precipitation: the opposite of dissolving! What do you see in the following clips: S 1043. mov S 1045. mov S 1046. mov S 1050. mov S 1057. mov S 1058. mov and S 1060. mov

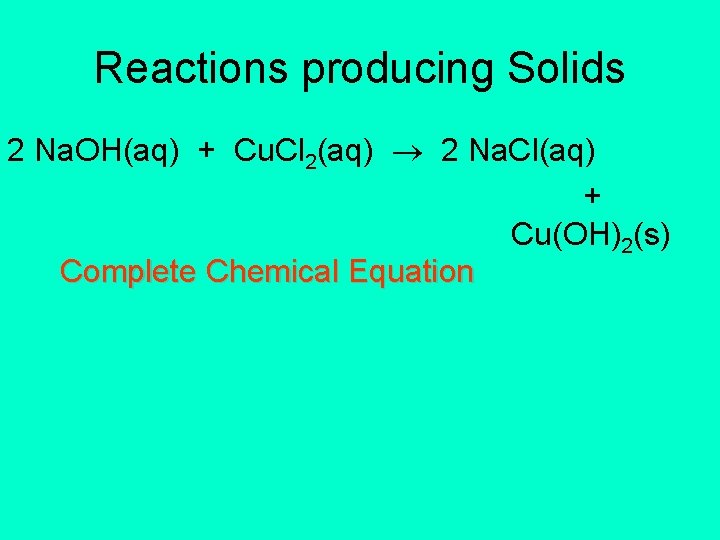
Reactions producing Solids 2 Na. OH(aq) + Cu. Cl 2(aq) 2 Na. Cl(aq) + Cu(OH)2(s) Complete Chemical Equation

Complete Ionic Equations 2 Na+(aq) + 2 OH-(aq) + Cu 2+(aq) + 2 Cl-(aq) 2 Na+(aq) + 2 Cl-(aq) + Cu(OH)2(s) Substances that are ions in solution are written as ions in solution.

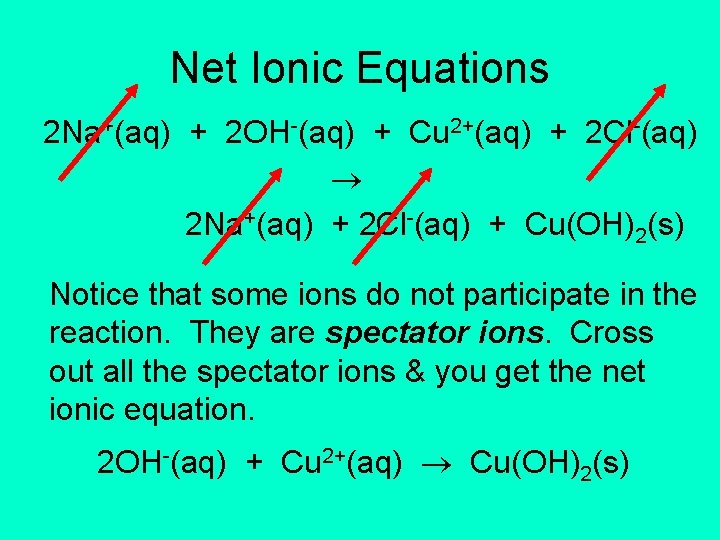
Net Ionic Equations 2 Na+(aq) + 2 OH-(aq) + Cu 2+(aq) + 2 Cl-(aq) 2 Na+(aq) + 2 Cl-(aq) + Cu(OH)2(s) Notice that some ions do not participate in the reaction. They are spectator ions. Cross out all the spectator ions & you get the net ionic equation. 2 OH-(aq) + Cu 2+(aq) Cu(OH)2(s)

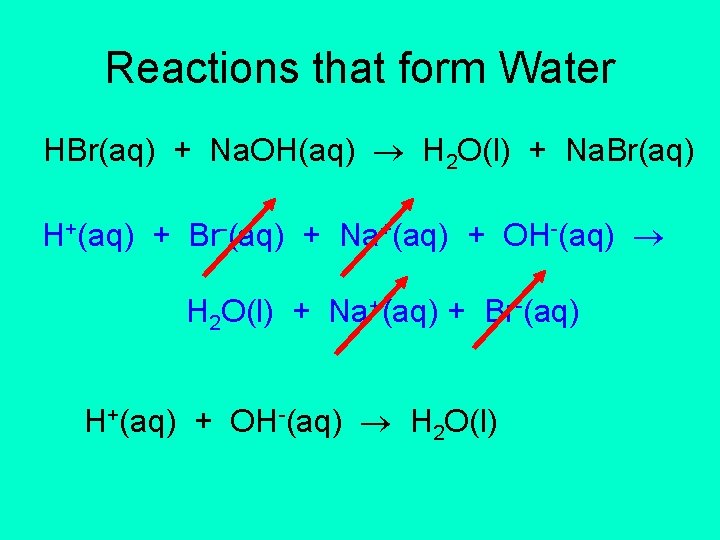
Reactions that form Water HBr(aq) + Na. OH(aq) H 2 O(l) + Na. Br(aq) H+(aq) + Br-(aq) + Na+(aq) + OH-(aq) H 2 O(l) + Na+(aq) + Br-(aq) H+(aq) + OH-(aq) H 2 O(l)

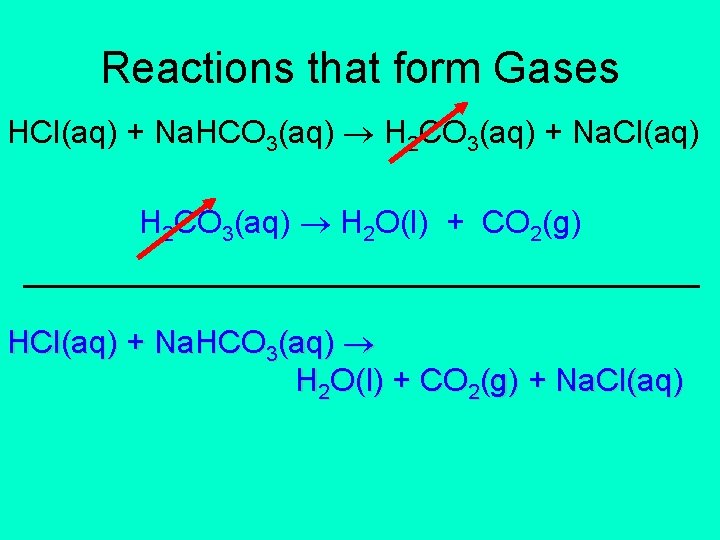
Reactions that form Gases HCl(aq) + Na. HCO 3(aq) H 2 CO 3(aq) + Na. Cl(aq) H 2 CO 3(aq) H 2 O(l) + CO 2(g) ___________________ HCl(aq) + Na. HCO 3(aq) H 2 O(l) + CO 2(g) + Na. Cl(aq)

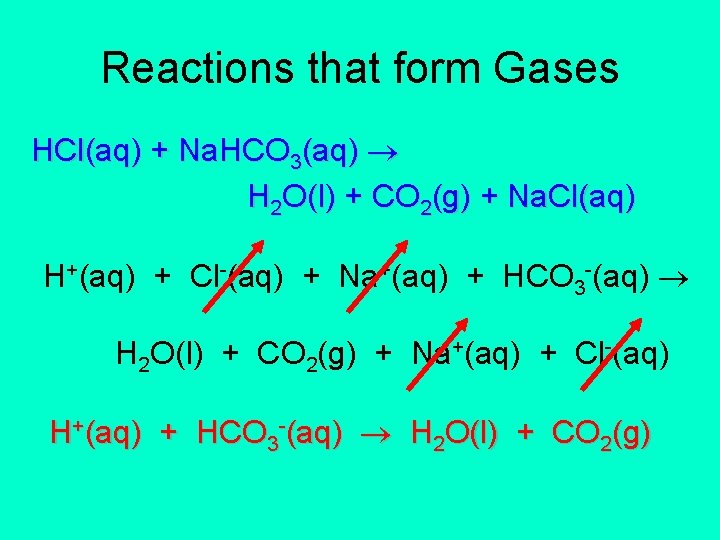
Reactions that form Gases HCl(aq) + Na. HCO 3(aq) H 2 O(l) + CO 2(g) + Na. Cl(aq) H+(aq) + Cl-(aq) + Na+(aq) + HCO 3 -(aq) H 2 O(l) + CO 2(g) + Na+(aq) + Cl-(aq) H+(aq) + HCO 3 -(aq) H 2 O(l) + CO 2(g)

Conservation of Charge • Total charge on reactant side must equal total charge on product side.