Displacement Reactions Single Displacement Reactions Single Displacement reaction


- Slides: 15

Displacement Reactions

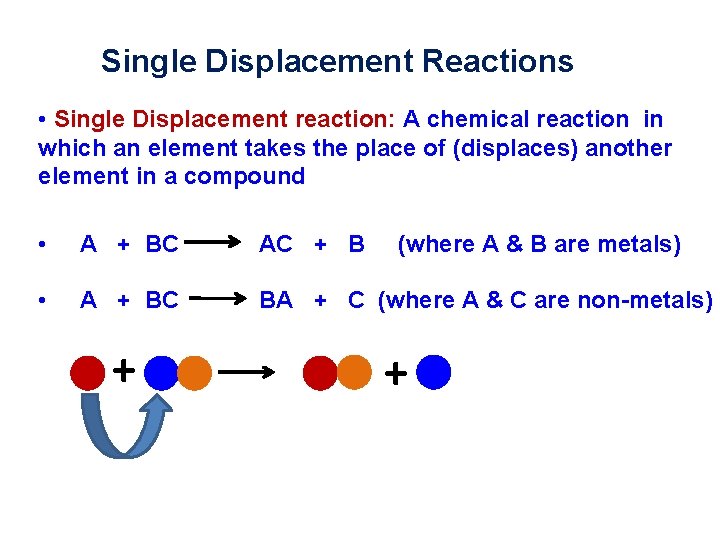
Single Displacement Reactions • Single Displacement reaction: A chemical reaction in which an element takes the place of (displaces) another element in a compound • A + BC AC + B • A + BC BA + C (where A & C are non-metals) + (where A & B are metals) +

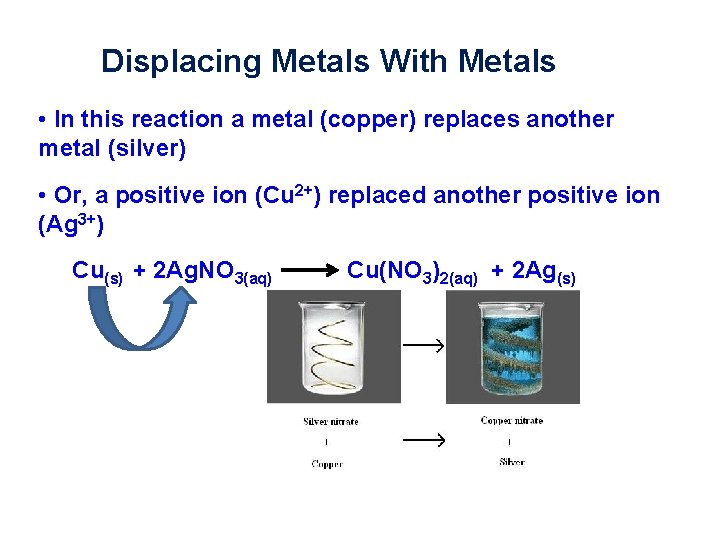
Displacing Metals With Metals • When a piece of copper is placed into silver nitrate, metallic silver ions form on the copper • The solution turns blue because copper atoms from the wire dissolve in solution and displaces the silver from the silver nitrate compound Cu(s) + 2 Ag. NO 3(aq) Cu(NO 3)2(aq) + 2 Ag(s)

Displacing Metals With Metals • In this reaction a metal (copper) replaces another metal (silver) • Or, a positive ion (Cu 2+) replaced another positive ion (Ag 3+) Cu(s) + 2 Ag. NO 3(aq) Cu(NO 3)2(aq) + 2 Ag(s)

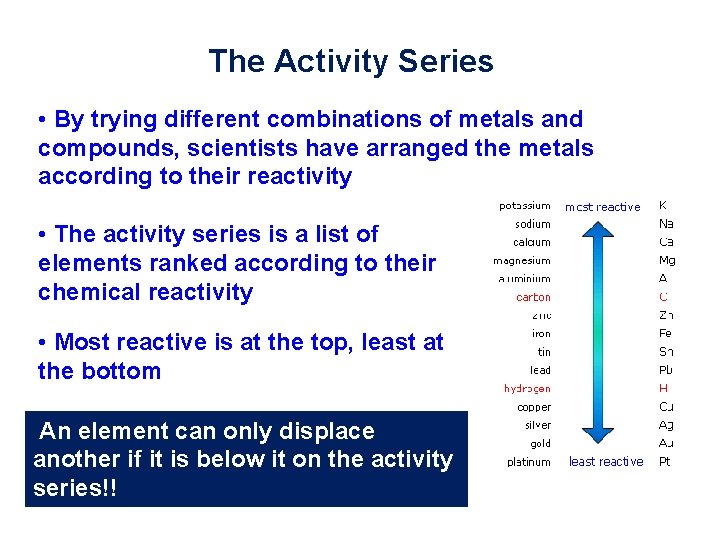
The Activity Series • By trying different combinations of metals and compounds, scientists have arranged the metals according to their reactivity • The activity series is a list of elements ranked according to their chemical reactivity • Most reactive is at the top, least at the bottom An element can only displace another if it is below it on the activity series!!

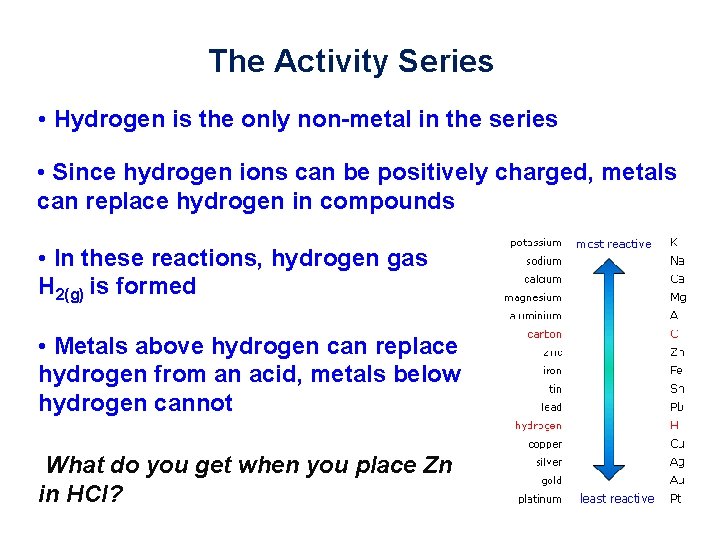
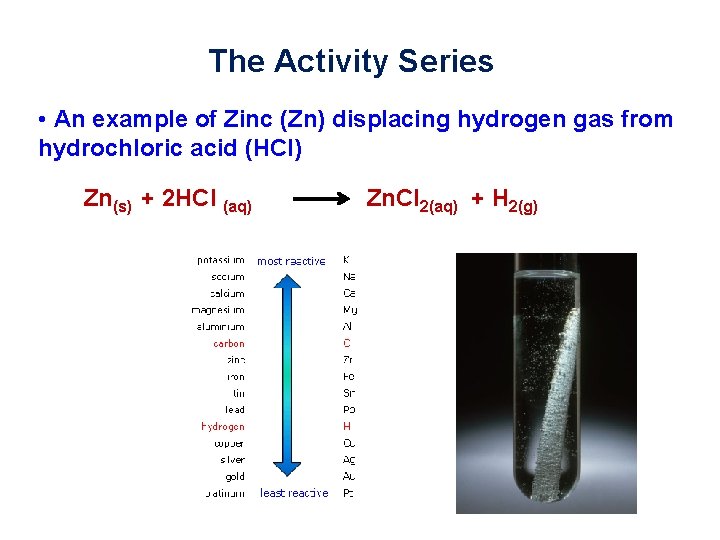
The Activity Series • Hydrogen is the only non-metal in the series • Since hydrogen ions can be positively charged, metals can replace hydrogen in compounds • In these reactions, hydrogen gas H 2(g) is formed • Metals above hydrogen can replace hydrogen from an acid, metals below hydrogen cannot What do you get when you place Zn in HCl?

The Activity Series • An example of Zinc (Zn) displacing hydrogen gas from hydrochloric acid (HCl) Zn(s) + 2 HCl (aq) Zn. Cl 2(aq) + H 2(g)

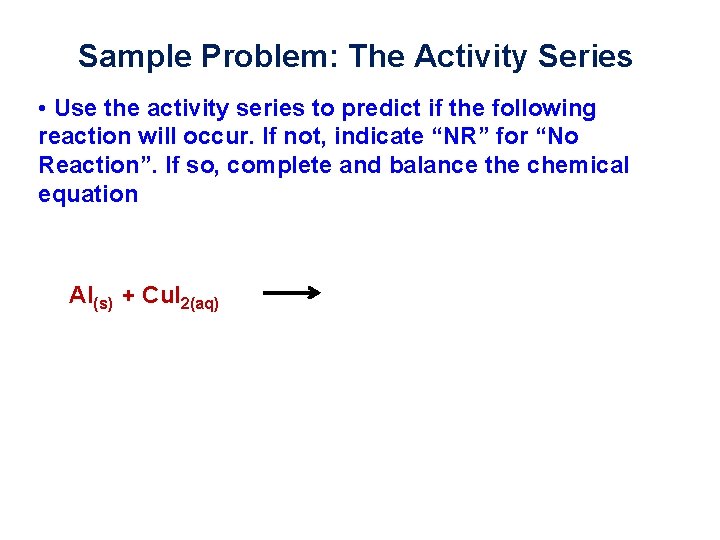
Sample Problem: The Activity Series • Use the activity series to predict if the following reaction will occur. If not, indicate “NR” for “No Reaction”. If so, complete and balance the chemical equation Al(s) + Cu. I 2(aq)

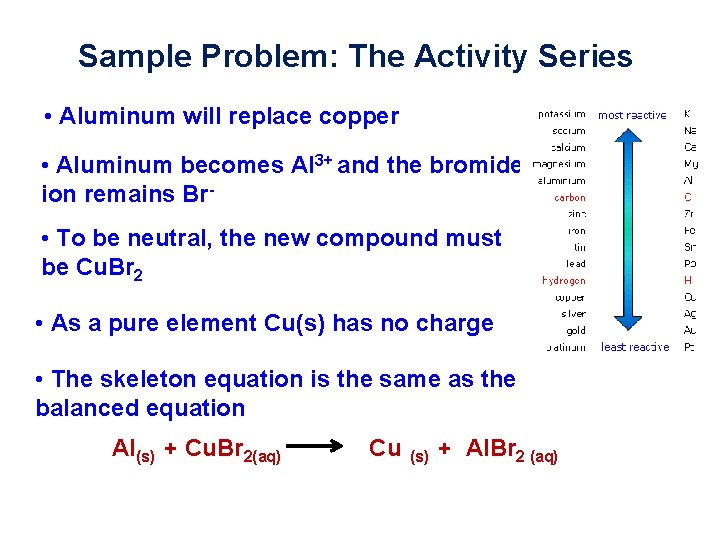
Sample Problem: The Activity Series • Aluminum will replace copper • Aluminum becomes Al 3+ and the bromide ion remains Br • To be neutral, the new compound must be Cu. Br 2 • As a pure element Cu(s) has no charge • The skeleton equation is the same as the balanced equation Al(s) + Cu. Br 2(aq) Cu (s) + Al. Br 2 (aq)

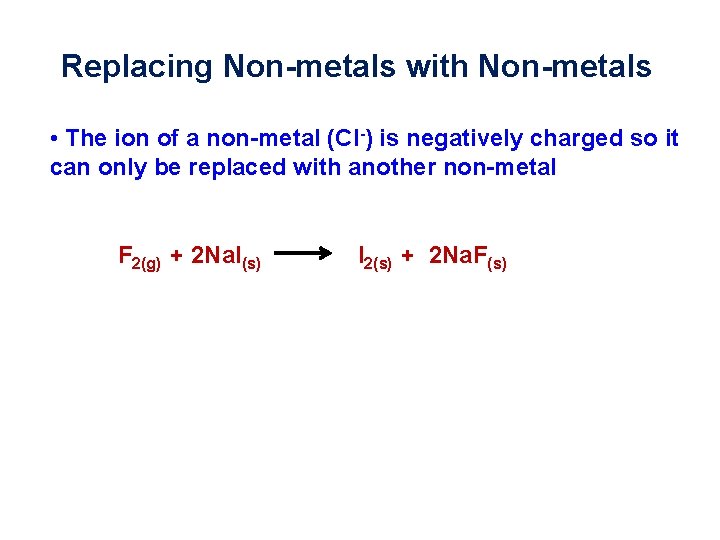
Replacing Non-metals with Non-metals • The ion of a non-metal (Cl-) is negatively charged so it can only be replaced with another non-metal F 2(g) + 2 Na. I(s) I 2(s) + 2 Na. F(s)

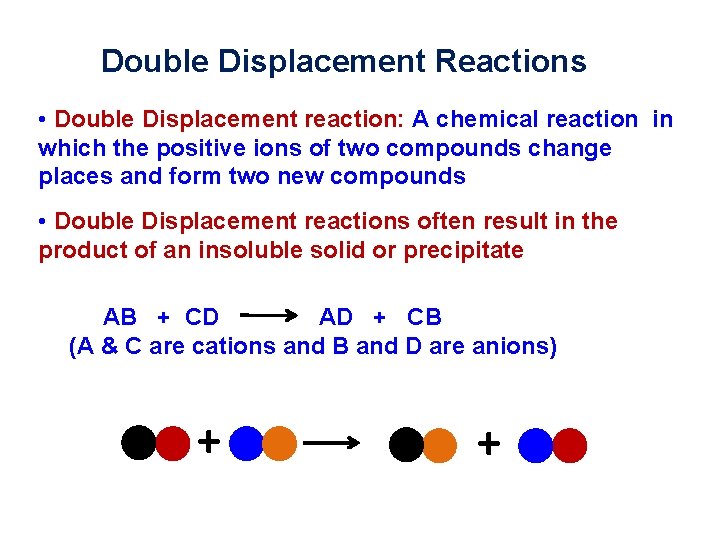
Double Displacement Reactions • Double Displacement reaction: A chemical reaction in which the positive ions of two compounds change places and form two new compounds • Double Displacement reactions often result in the product of an insoluble solid or precipitate AB + CD AD + CB (A & C are cations and B and D are anions) + +

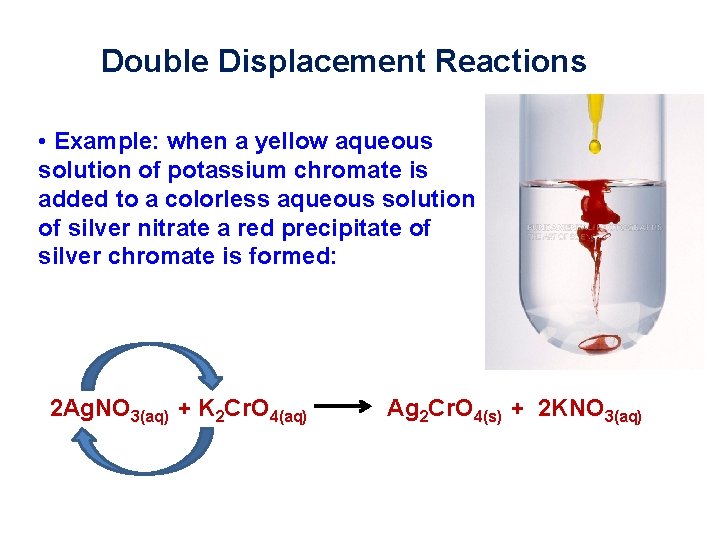
Double Displacement Reactions • Example: when a yellow aqueous solution of potassium chromate is added to a colorless aqueous solution of silver nitrate a red precipitate of silver chromate is formed: 2 Ag. NO 3(aq) + K 2 Cr. O 4(aq) Ag 2 Cr. O 4(s) + 2 KNO 3(aq)

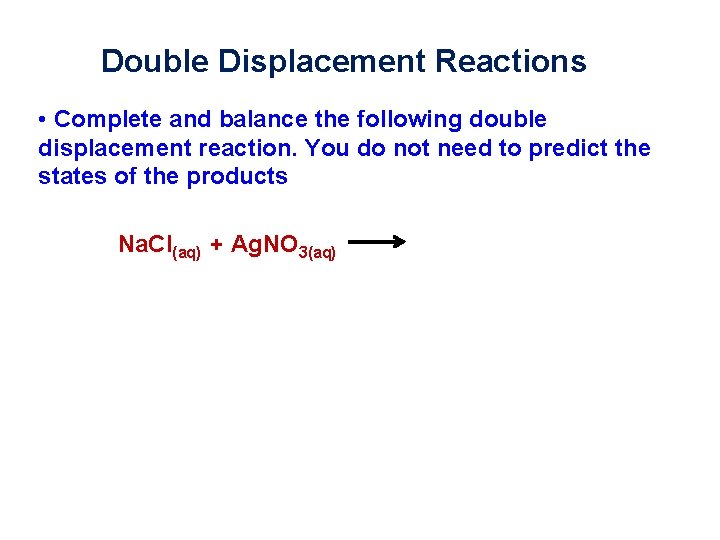
Double Displacement Reactions • Complete and balance the following double displacement reaction. You do not need to predict the states of the products Na. Cl(aq) + Ag. NO 3(aq)

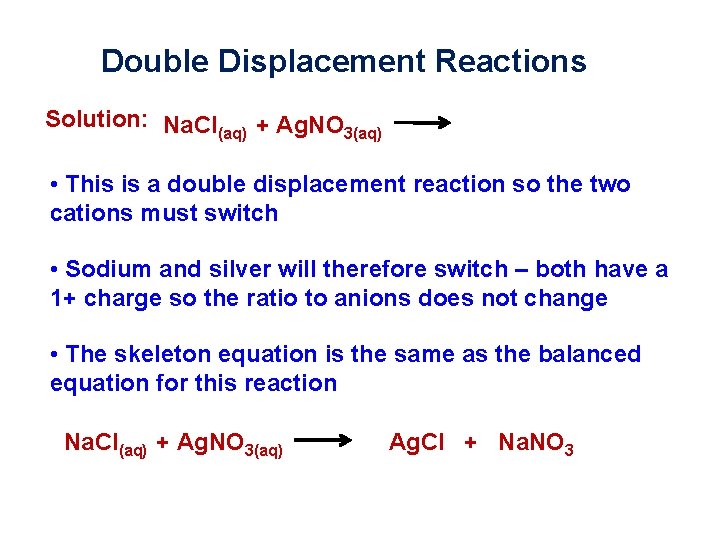
Double Displacement Reactions Solution: Na. Cl(aq) + Ag. NO 3(aq) • This is a double displacement reaction so the two cations must switch • Sodium and silver will therefore switch – both have a 1+ charge so the ratio to anions does not change • The skeleton equation is the same as the balanced equation for this reaction Na. Cl(aq) + Ag. NO 3(aq) Ag. Cl + Na. NO 3

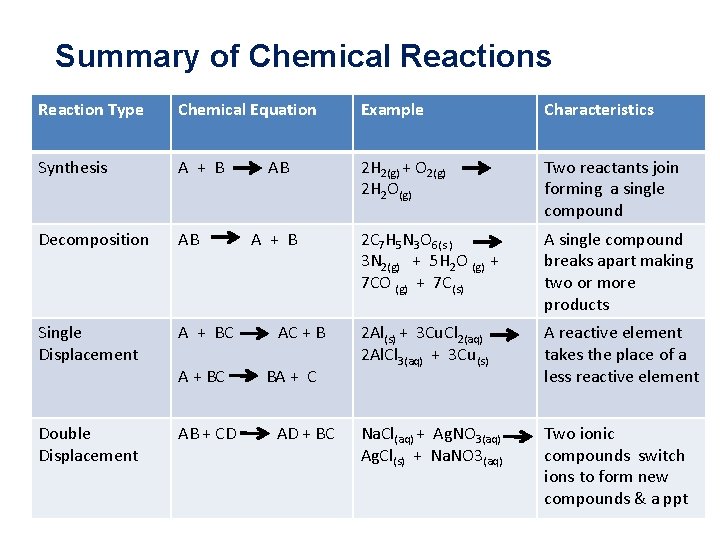
Summary of Chemical Reactions Reaction Type Chemical Equation Example Characteristics Synthesis A + B 2 H 2(g) + O 2(g) 2 H 2 O(g) Two reactants join forming a single compound Decomposition AB 2 C 7 H 5 N 3 O 6(s ) 3 N 2(g) + 5 H 2 O (g) + 7 C(s) A single compound breaks apart making two or more products Single Displacement A + BC 2 Al(s) + 3 Cu. Cl 2(aq) 2 Al. Cl 3(aq) + 3 Cu(s) A reactive element takes the place of a less reactive element Double Displacement AB + CD Na. Cl(aq) + Ag. NO 3(aq) Ag. Cl(s) + Na. NO 3(aq) Two ionic compounds switch ions to form new compounds & a ppt A + BC AB A + B AC + B BA + C AD + BC
 Single displacement vs double displacement
Single displacement vs double displacement Decomposition reaction cartoon
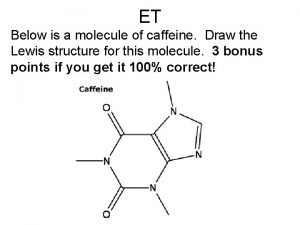
Decomposition reaction cartoon Draw the structure of caffeine
Draw the structure of caffeine Single replacement reaction general equation
Single replacement reaction general equation 20 examples of redox reaction
20 examples of redox reaction Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Reactivity series and displacement reactions
Reactivity series and displacement reactions 10 example of displacement reaction
10 example of displacement reaction Precipitation reaction
Precipitation reaction Displacement reaction experiment
Displacement reaction experiment Electron pair displacement reaction
Electron pair displacement reaction Half life formula
Half life formula E1cb elimination reaction
E1cb elimination reaction