Single Displacement Reactions Single Displacement Reactions Singlereplacement reaction


- Slides: 11

Single Displacement Reactions

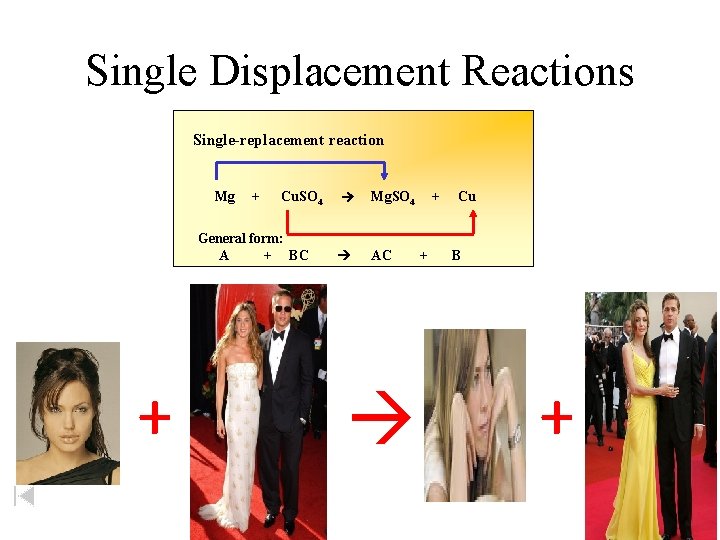
Single Displacement Reactions Single-replacement reaction Mg + Cu. SO 4 General form: A + BC + Mg. SO 4 AC + + Cu B +

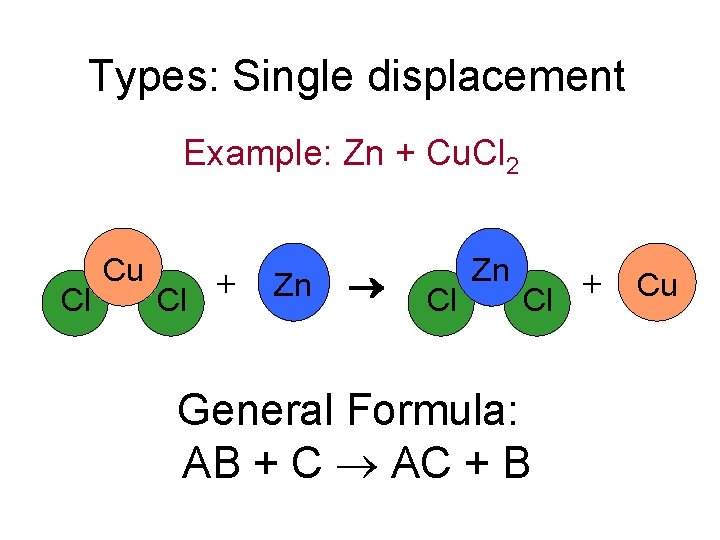
Types: Single displacement Example: Zn + Cu. Cl 2 Cl Cu + Cl Zn + Cu Cl General Formula: AB + C AC + B

ONLY FOR SINGLE DISPL’M

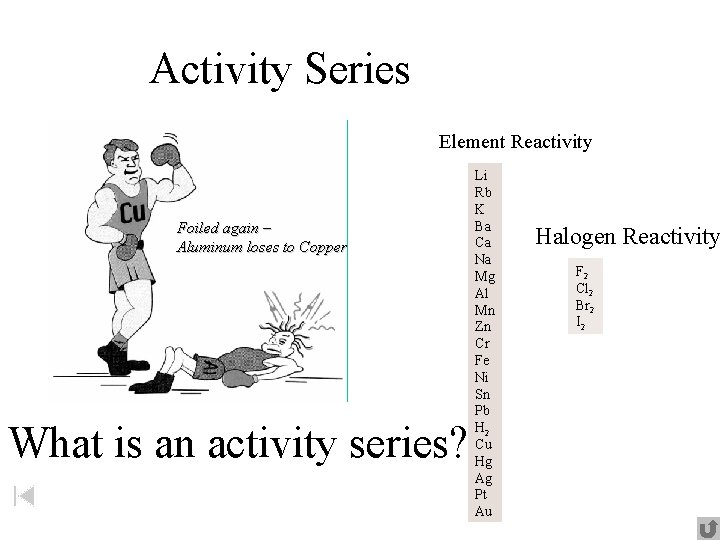
Activity Series Element Reactivity Foiled again – Aluminum loses to Copper What is an activity series? Li Rb K Ba Ca Na Mg Al Mn Zn Cr Fe Ni Sn Pb H 2 Cu Hg Ag Pt Au Halogen Reactivity F 2 Cl 2 Br 2 I 2

The activity series ranks the relative reactivity of metals. It allows us to predict if certain chemicals No, Ni is will undergo single displacement reactions when Yes, Li is below Na mixed: metals near the top are most Zn reactive above and will displacing metals near the bottom. Q: Which of these will react? Yes, Al is Cu Fe + Cu. SO 4 Cu + Feabove 2(SO 4)3 Ni + Na. Cl NR (no reaction) Yes, Fe is Li + Zn. CO 3 Zn + Li CO 2 3 Al + Cu. Cl 2 Cu + Al. Clabove Cu 3 K Na Li Ca Mg Al Zn Fe Ni Sn Pb H Cu Hg Ag Au

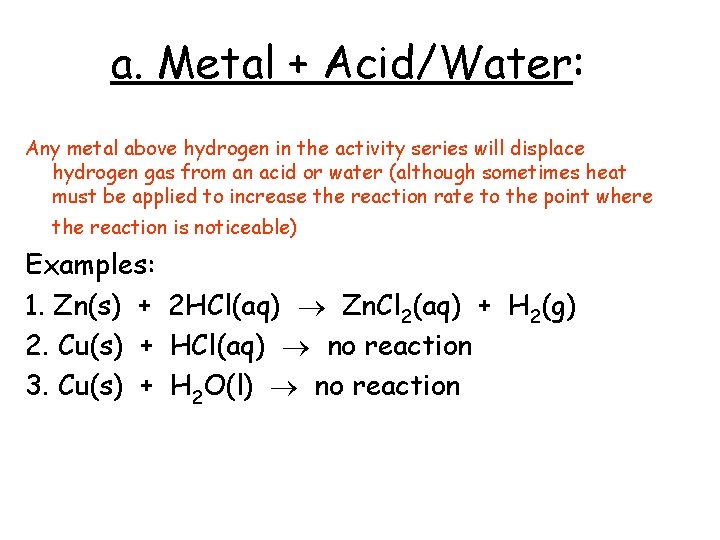
a. Metal + Acid/Water: Any metal above hydrogen in the activity series will displace hydrogen gas from an acid or water (although sometimes heat must be applied to increase the reaction rate to the point where the reaction is noticeable) Examples: 1. Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2(g) 2. Cu(s) + HCl(aq) no reaction 3. Cu(s) + H 2 O(l) no reaction

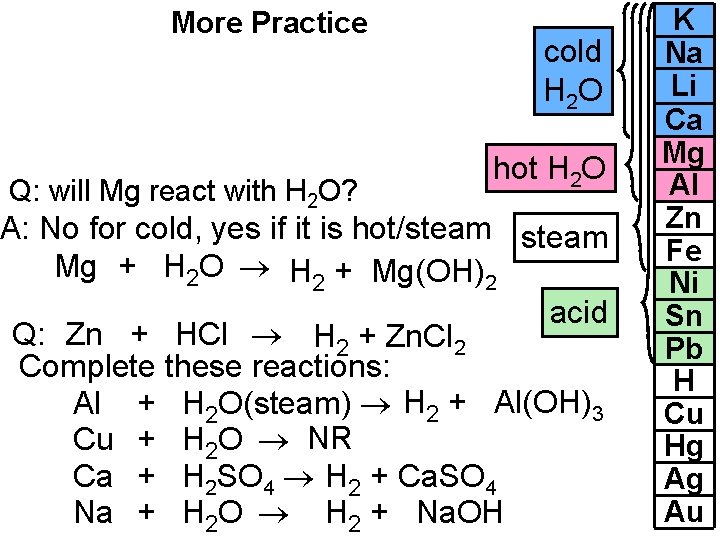
More Practice Q: will Mg react with H 2 O? cold H 2 O hot H 2 O A: No for cold, yes if it is hot/steam Mg + H 2 O H 2 + Mg(OH)2 acid Q: Zn + HCl H 2 + Zn. Cl 2 Complete these reactions: Al + H 2 O(steam) H 2 + Al(OH)3 Cu + H 2 O NR Ca + H 2 SO 4 H 2 + Ca. SO 4 Na + H 2 O H 2 + Na. OH K Na Li Ca Mg Al Zn Fe Ni Sn Pb H Cu Hg Ag Au

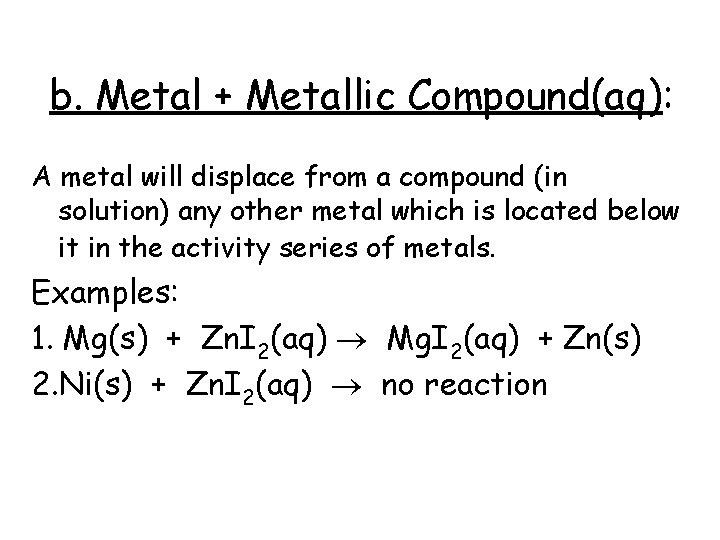
b. Metal + Metallic Compound(aq): A metal will displace from a compound (in solution) any other metal which is located below it in the activity series of metals. Examples: 1. Mg(s) + Zn. I 2(aq) Mg. I 2(aq) + Zn(s) 2. Ni(s) + Zn. I 2(aq) no reaction

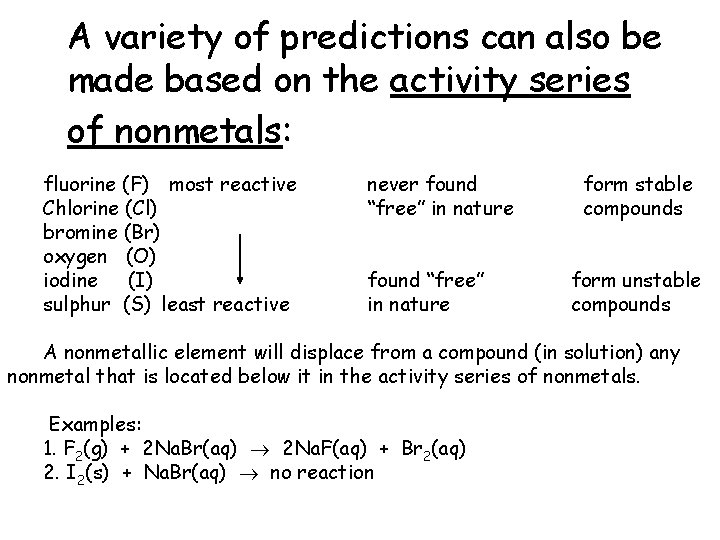
A variety of predictions can also be made based on the activity series of nonmetals: fluorine (F) most reactive Chlorine (Cl) bromine (Br) oxygen (O) iodine (I) sulphur (S) least reactive never found “free” in nature form stable compounds form unstable compounds A nonmetallic element will displace from a compound (in solution) any nonmetal that is located below it in the activity series of nonmetals. Examples: 1. F 2(g) + 2 Na. Br(aq) 2 Na. F(aq) + Br 2(aq) 2. I 2(s) + Na. Br(aq) no reaction

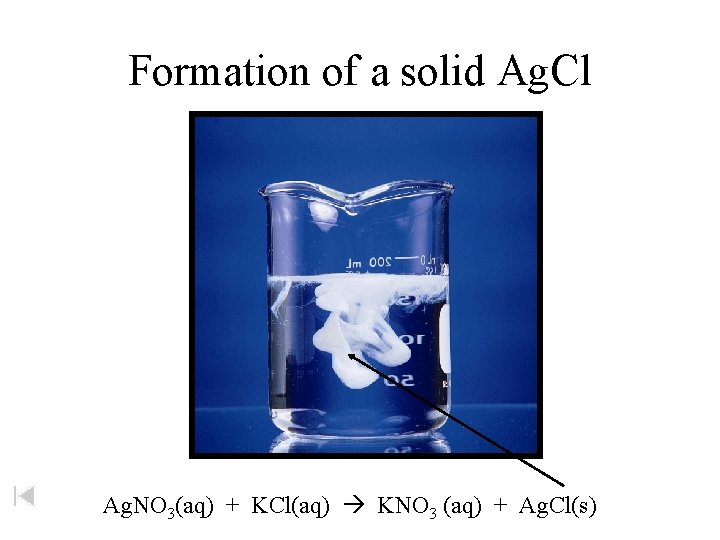
Formation of a solid Ag. Cl Ag. NO 3(aq) + KCl(aq) KNO 3 (aq) + Ag. Cl(s)