Types of Reactions The Six Different Types of


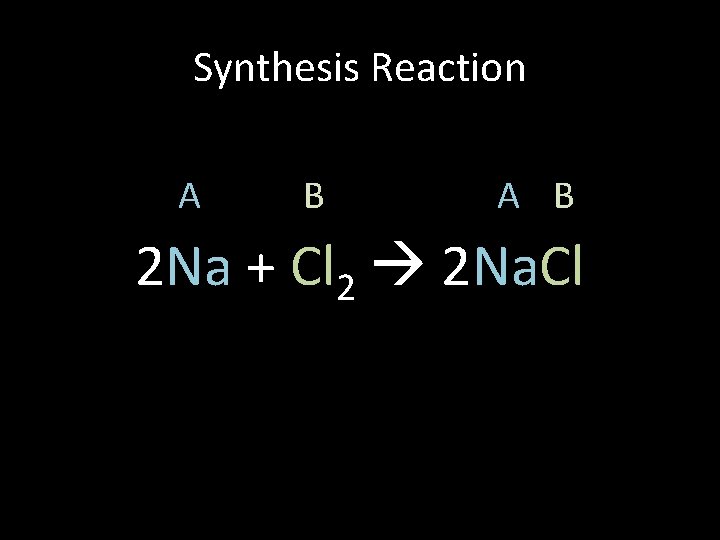

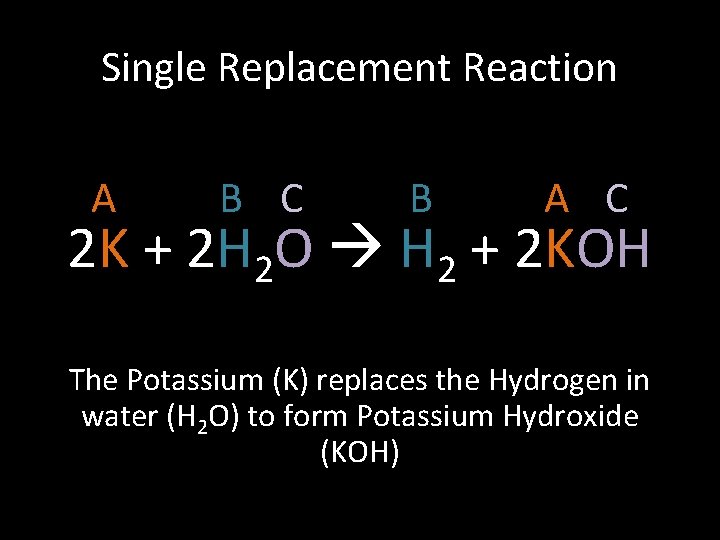


- Slides: 14

Types of Reactions

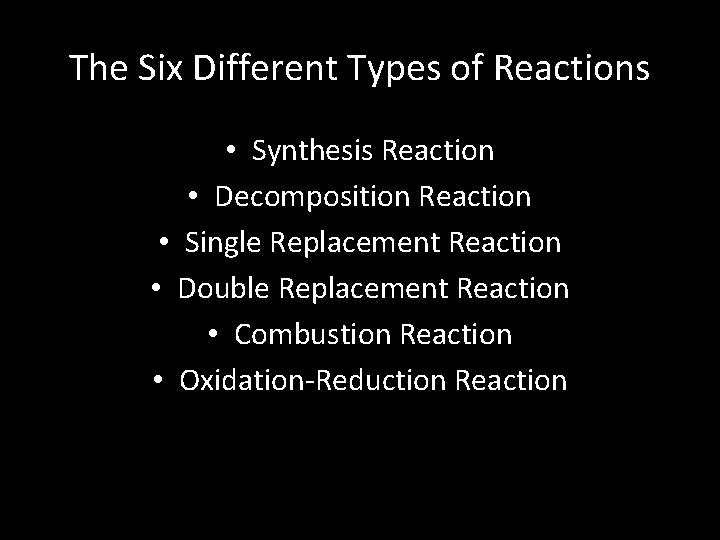
The Six Different Types of Reactions • Synthesis Reaction • Decomposition Reaction • Single Replacement Reaction • Double Replacement Reaction • Combustion Reaction • Oxidation-Reduction Reaction

Synthesis Reaction • A reaction where two or more substances react to produce one substance • The reactants can be either elements or compounds • A + B AB
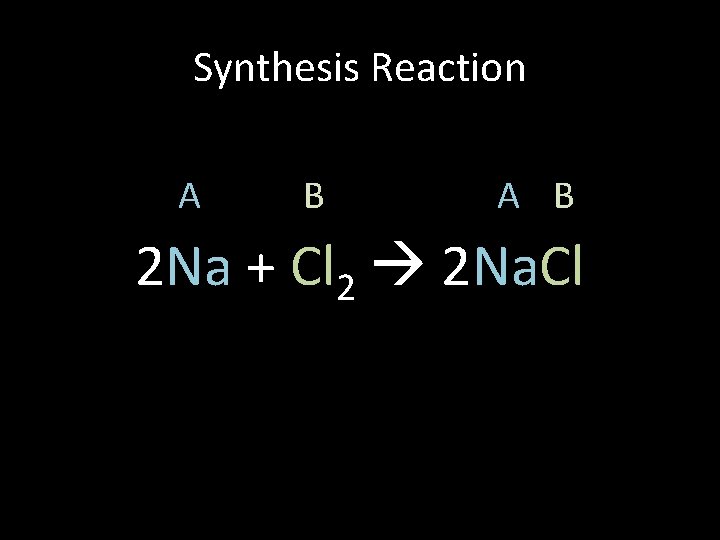
Synthesis Reaction A B 2 Na + Cl 2 2 Na. Cl

Decomposition Reaction • A reaction in which a compound breaks down into two or more simpler substances • The products may be elements or compounds • AB A + B

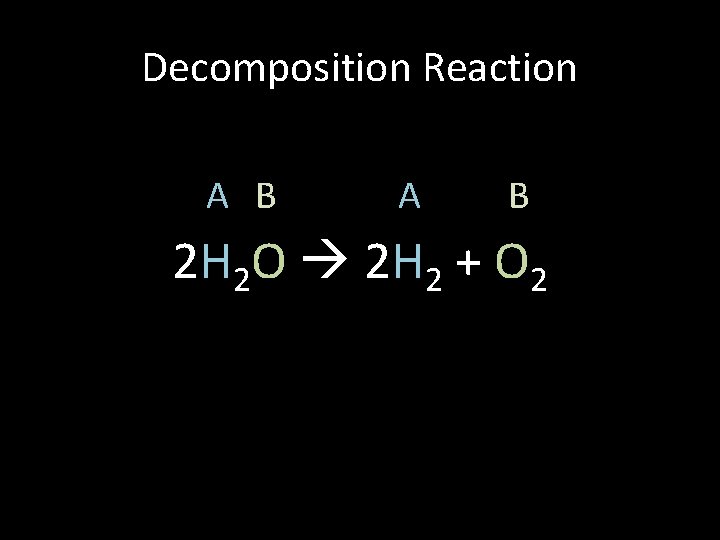
Decomposition Reaction A B 2 H 2 O 2 H 2 + O 2

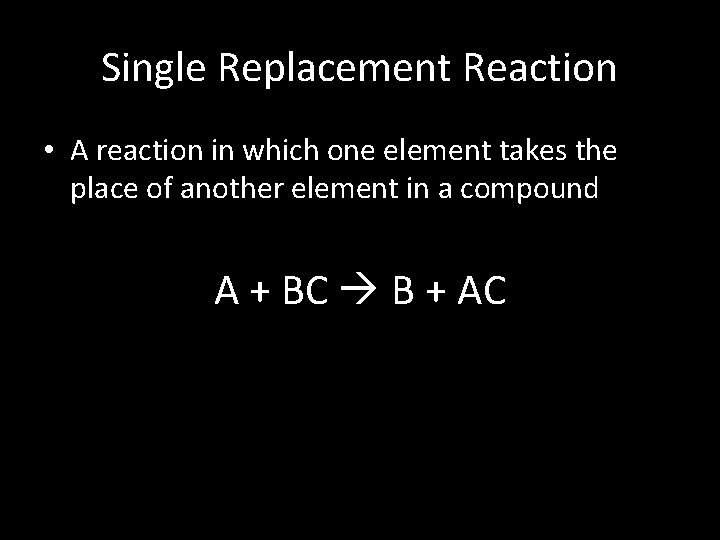
Single Replacement Reaction • A reaction in which one element takes the place of another element in a compound A + BC B + AC
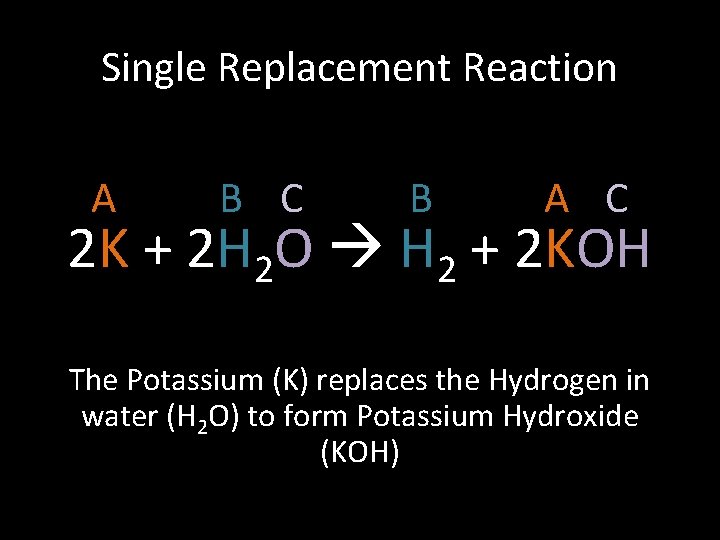
Single Replacement Reaction A B C B A C 2 K + 2 H 2 O H 2 + 2 KOH The Potassium (K) replaces the Hydrogen in water (H 2 O) to form Potassium Hydroxide (KOH)

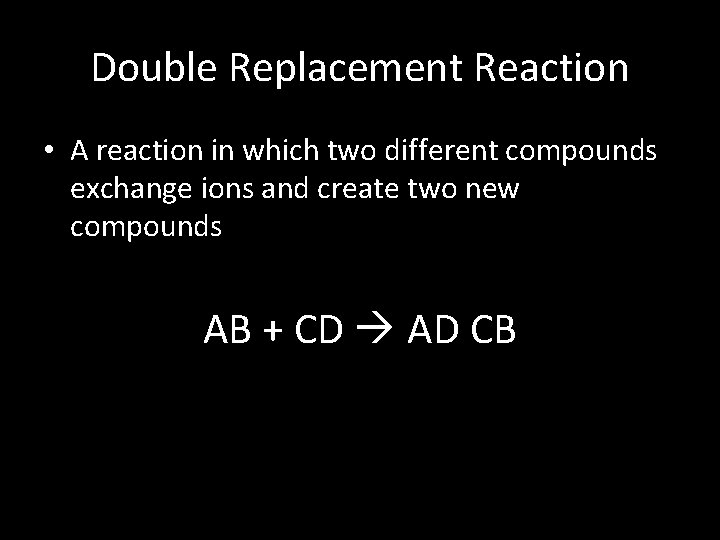
Double Replacement Reaction • A reaction in which two different compounds exchange ions and create two new compounds AB + CD AD CB

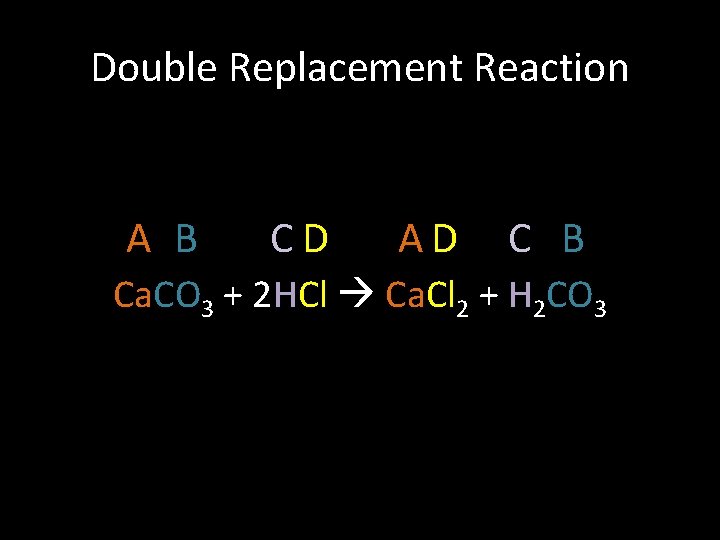
Double Replacement Reaction A B CD AD C B Ca. CO 3 + 2 HCl Ca. Cl 2 + H 2 CO 3

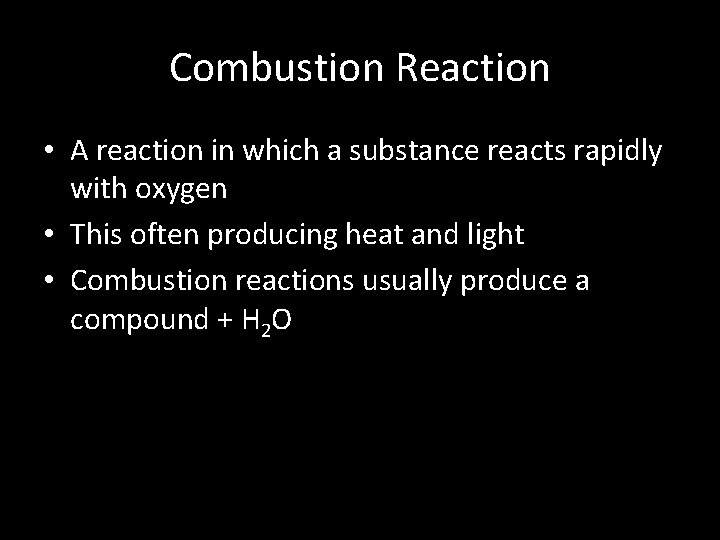
Combustion Reaction • A reaction in which a substance reacts rapidly with oxygen • This often producing heat and light • Combustion reactions usually produce a compound + H 2 O

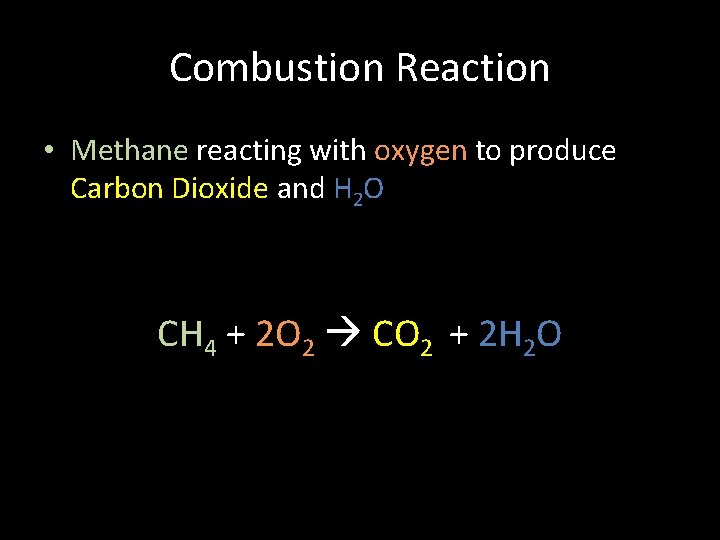
Combustion Reaction • Methane reacting with oxygen to produce Carbon Dioxide and H 2 O CH 4 + 2 O 2 CO 2 + 2 H 2 O

Oxidation - Reduction • Oxidation – Reduction reactions involve an atom or molecule losing or gaining an electron • Usually involves a metal and oxygen

Oxidation - Reduction • The Oxidation-Reduction reaction between Iron and Oxygen is often known as Rust
 Balancing redox reactions
Balancing redox reactions Different types of reactions
Different types of reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Redox reaction examples
Redox reaction examples Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers A polygon with six congruent sides and six congruent angles
A polygon with six congruent sides and six congruent angles What do chess pieces represent
What do chess pieces represent Thermosoftening plastics examples
Thermosoftening plastics examples Technicolor test
Technicolor test Sound will travel at different speeds in different mediums.
Sound will travel at different speeds in different mediums. Sound will travel at different speeds in different mediums.
Sound will travel at different speeds in different mediums. Different culture have different moral codes
Different culture have different moral codes Different angle different story
Different angle different story