Chemical Reactions Types of Reactions There are six
























- Slides: 37

Chemical Reactions

Types of Reactions • There are six types of chemical reactions we will talk about: 1. 2. 3. 4. 5. 6. • Combustion reactions Synthesis reactions Decomposition reactions Single displacement reactions Double displacement reactions Acid- Base neutralization reactions You need to be able to identify the type of reaction and predict the product(s)

Steps to Writing Reactions • Some steps for doing reactions 1. 2. 3. Identify the type of reaction Predict the product(s) using the type of reaction as a model Balance it Don’t forget about the diatomic elements! (Hockey Stick and Puck) For example, Oxygen is O 2. In a compound, it can’t be a diatomic element because it’s not an element anymore, it’s a compound!

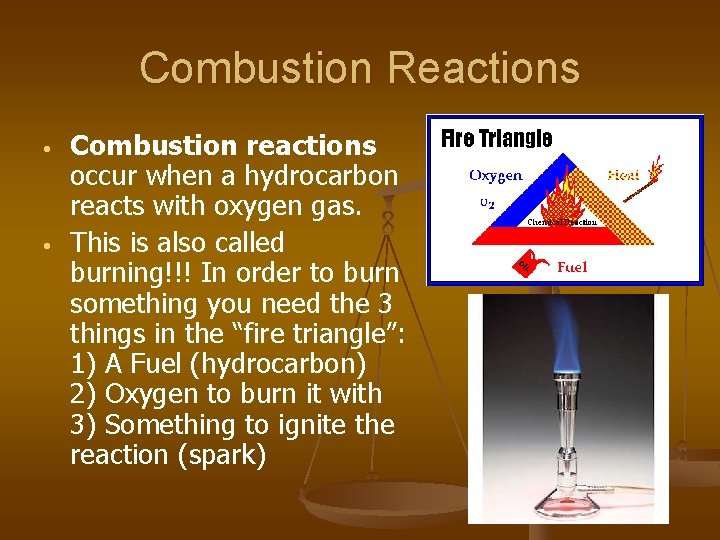
Combustion Reactions • • Combustion reactions occur when a hydrocarbon reacts with oxygen gas. This is also called burning!!! In order to burn something you need the 3 things in the “fire triangle”: 1) A Fuel (hydrocarbon) 2) Oxygen to burn it with 3) Something to ignite the reaction (spark)

Combustion Reactions • • • In general: Cx. Hy + O 2 CO 2 + H 2 O Products in combustion are ALWAYS carbon dioxide and water. (although incomplete burning does cause some byproducts like carbon monoxide) Combustion is used to heat homes and run automobiles (octane, as in gasoline, is C 8 H 18)

Combustion Reactions Edgar Allen Poe’s drooping eyes and mouth are potential signs of CO poisoning.

Combustion • Example • • C 5 H 12 + 8 O 2 5 CO 2 + 6 H 2 O Write the products and balance the following combustion reaction: • 11 H 2 O 2 C 10 H 22 +31 O 2 20 10 CO 2 +22

Hydrocarbons combust with oxygen to form carbon dioxide and water. Cx. Hy + O 2 → CO 2 + H 2 O

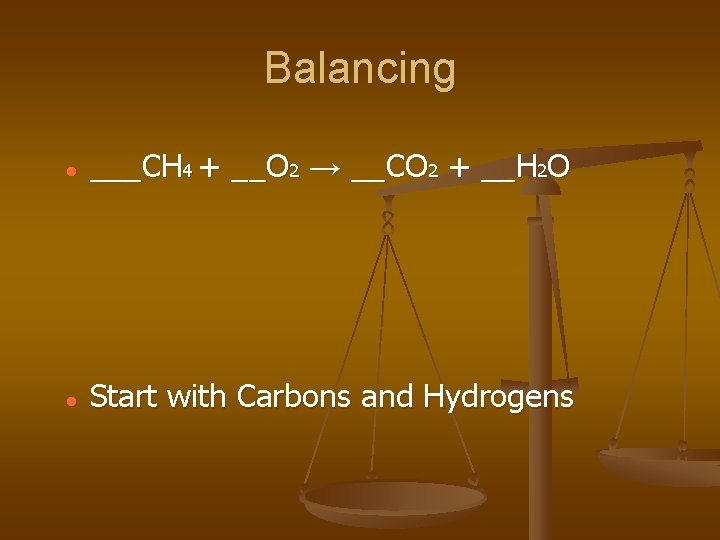
Balancing ___CH 4 + __O 2 → __CO 2 + __H 2 O Start with Carbons and Hydrogens

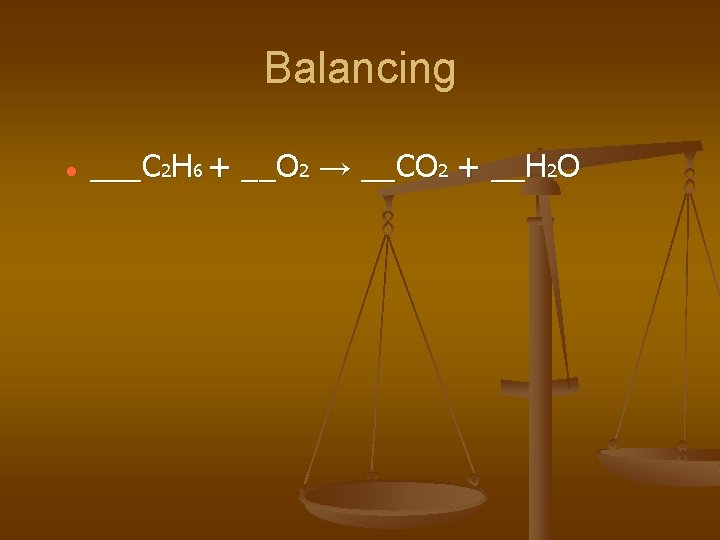
Balancing ___C 2 H 6 + __O 2 → __CO 2 + __H 2 O

Incomplete Combustion Not enough oxygen to completely react with hydrocarbon. Causes undesirable byproducts like carbon monoxide and elemental carbon. Can be reduced with improved design of engine to increase amount of oxygen in system.

Neutralization Reactions n n When an acid and base are mixed, they react and can neutralize each other. A neutralization reaction is a reaction between an acid and a base to form a salt and water. n ACID + BASE --> SALT + WATER n

n For example, hydrochloric acid and sodium hydroxide react as shown in the balanced equation below. n HCl + Na. OH --> Na. Cl + H 2 O n n n The salt that is formed in this reaction is sodium chloride, which is soluble in water. In most cases, the salt formed by a neutralization reaction is soluble in water. If the salt is insoluble, then a precipitate will form.

Example #1: n When sulphuric acid reacts with sodium hydroxide, sodium sulphate and water are produced. What is the balanced chemical equation for this neutralization reaction? n 1) Write an equation for this reaction. n

Applications of Neutralization Reactions n n Neutralization reactions have many commercial uses. Neutralization reactions are used in medicine. For example, acid reflux is a condition in which stomach acid causes discomfort. The symptoms can treated with antacids, which are composed of bases.

n n In agriculture, calcium carbonate (a base) can be be added to acidic soil to neutralize it. In the food industry, neutralization reactions are used to adjust the p. H of products. For example, vinegar is often added to packaged foods, such as ketchup, for taste and to prevent harmful bacteria from growing and causing them to spoil.

Synthesis reactions • • Synthesis reactions occur when two substances (generally elements) combine and form a compound. (Sometimes these are called combination or addition reactions. ) reactant + reactant 1 product Basically: A + B AB • • Example: 2 H 2 + O 2 2 H 2 O Example: C + O 2 CO 2

Synthesis Reactions • Here is another example of a synthesis reaction

Practice • • Predict the products. Write and balance the following synthesis reaction equations. Sodium metal reacts with chlorine gas 2 Na(s) + Cl 2(g) 2 Na. Cl(s) Solid Magnesium reacts with fluorine gas Mg(s) + F 2(g) Mg. F 2(s) Aluminum metal reacts with fluorine gas 2 Al(s) + 3 F 2(g) 2 Al. F 3(s)

Decomposition RXN

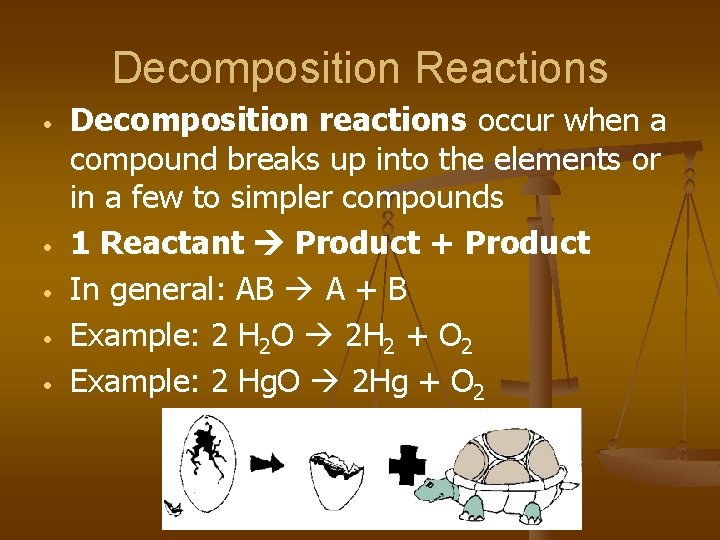
Decomposition Reactions • • • Decomposition reactions occur when a compound breaks up into the elements or in a few to simpler compounds 1 Reactant Product + Product In general: AB A + B Example: 2 H 2 O 2 H 2 + O 2 Example: 2 Hg. O 2 Hg + O 2

Decomposition Reactions • Another view of a decomposition reaction:

Decomposition Exceptions • Carbonates and chlorates are special case decomposition reactions that do not go to the elements. • Carbonates (CO 32 -) decompose to carbon dioxide and a metal oxide • • Chlorates (Cl. O 3 -) decompose to oxygen gas and a metal chloride • • Example: Ca. CO 3 CO 2 + Ca. O Example: 2 Al(Cl. O 3)3 2 Al. Cl 3 + 9 O 2 There are other special cases, but we will not explore those in Chemistry I

Practice • • • Predict the products. Then, write and balance the following decomposition reaction equations: Solid Lead (IV) oxide decomposes Pb(s) + O 2(g) Aluminum nitride decomposes 2 Al(s) + N 2(g)

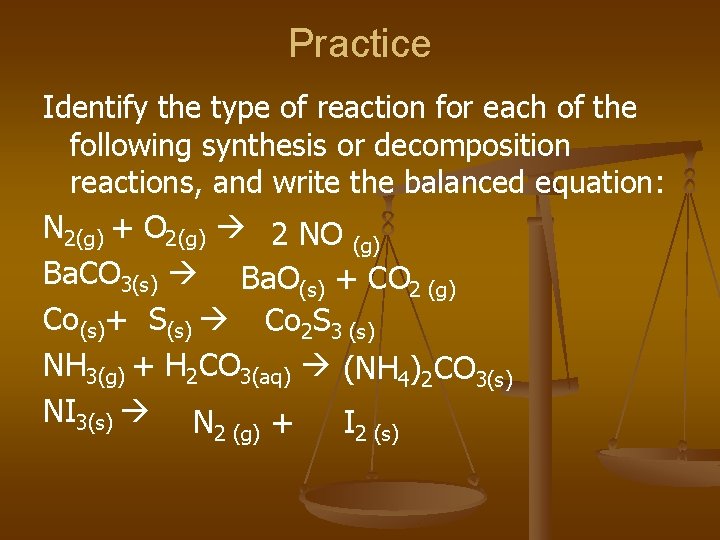
Practice Identify the type of reaction for each of the following synthesis or decomposition reactions, and write the balanced equation: N 2(g) + O 2(g) 2 NO (g) Ba. CO 3(s) Ba. O(s) + CO 2 (g) Co(s)+ S(s) Co 2 S 3 (s) NH 3(g) + H 2 CO 3(aq) (NH 4)2 CO 3(s) NI 3(s) N + I 2 (g) 2 (s)

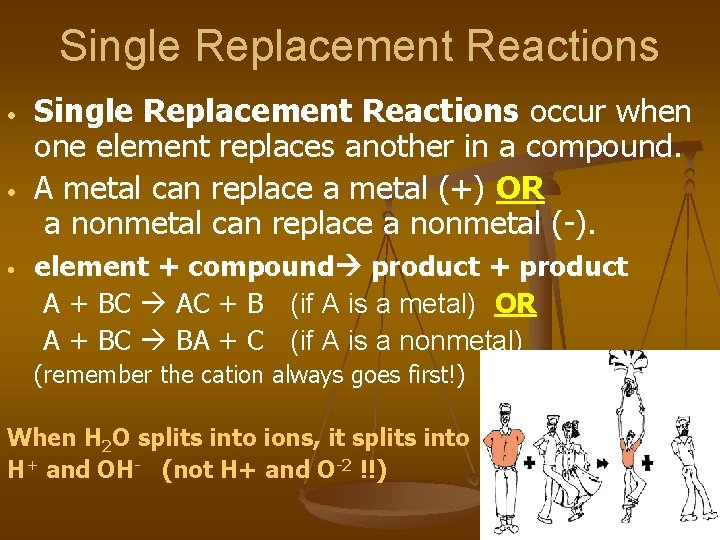
Single Replacement Reactions • • • Single Replacement Reactions occur when one element replaces another in a compound. A metal can replace a metal (+) OR a nonmetal can replace a nonmetal (-). element + compound product + product A + BC AC + B (if A is a metal) OR A + BC BA + C (if A is a nonmetal) (remember the cation always goes first!) When H 2 O splits into ions, it splits into H+ and OH- (not H+ and O-2 !!)

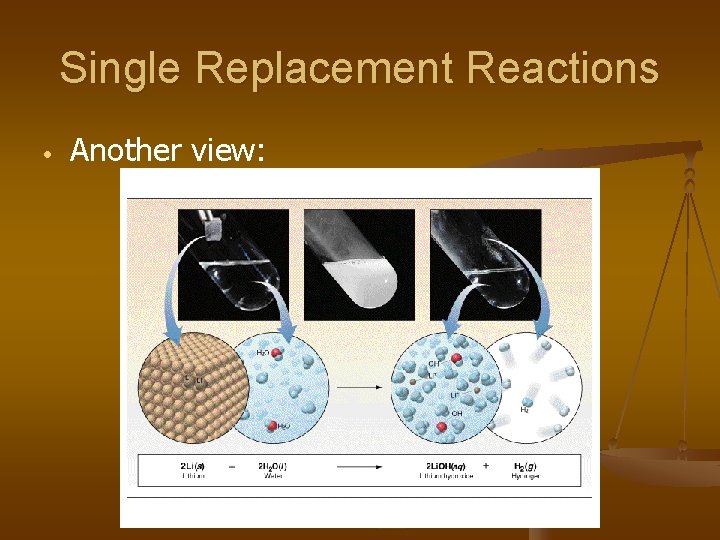
Single Replacement Reactions • Another view:

Single Replacement Reactions Write and balance the following single replacement reaction equation: • Zinc metal reacts with aqueous hydrochloric acid Zn(s) + 2 HCl(aq) Zn. Cl 2 + H 2(g) Note: Zinc replaces the hydrogen ion in the reaction •

Single Replacement Reactions • Sodium chloride solid reacts with fluorine gas 2 Na. Cl(s) + F 2(g) 2 Na. F(s) + Cl 2(g) Note that fluorine replaces chlorine in the compound • Aluminum metal reacts with aqueous copper (II) nitrate 2 Al(s)+ 3 Cu(NO 3)2(aq) 3 Cu(s) + 2 Al(NO 3)3(aq)

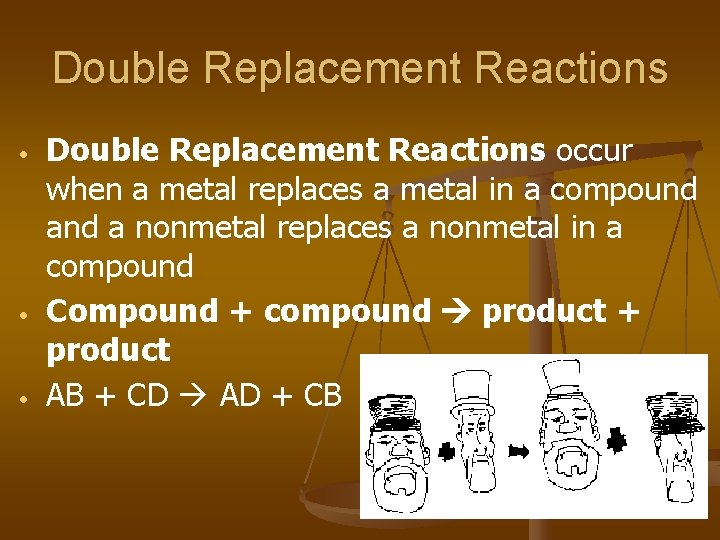
Double Replacement Reactions • • • Double Replacement Reactions occur when a metal replaces a metal in a compound a nonmetal replaces a nonmetal in a compound Compound + compound product + product AB + CD AD + CB

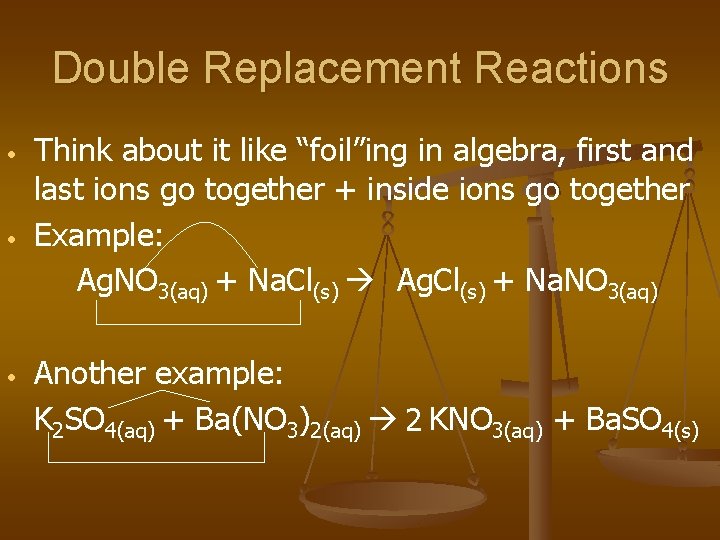
Double Replacement Reactions • • • Think about it like “foil”ing in algebra, first and last ions go together + inside ions go together Example: Ag. NO 3(aq) + Na. Cl(s) Ag. Cl(s) + Na. NO 3(aq) Another example: K 2 SO 4(aq) + Ba(NO 3)2(aq) 2 KNO 3(aq) + Ba. SO 4(s)

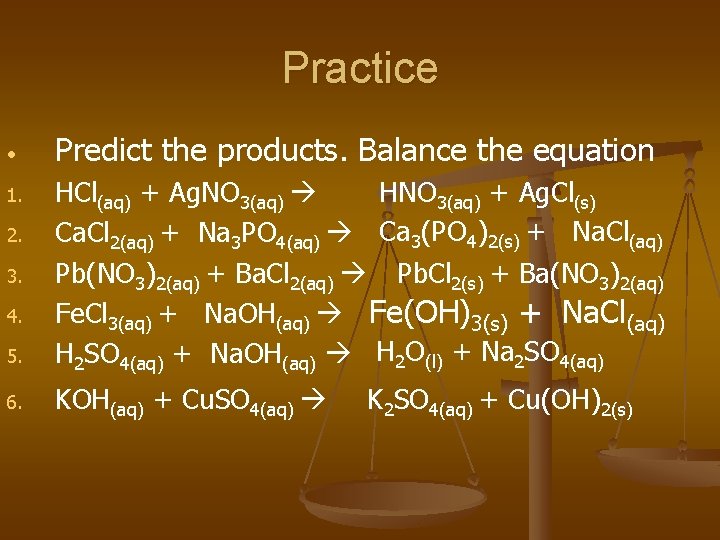
Practice • Predict the products. Balance the equation 5. HNO 3(aq) + Ag. Cl(s) HCl(aq) + Ag. NO 3(aq) Ca. Cl 2(aq) + Na 3 PO 4(aq) Ca 3(PO 4)2(s) + Na. Cl(aq) Pb(NO 3)2(aq) + Ba. Cl 2(aq) Pb. Cl 2(s) + Ba(NO 3)2(aq) Fe. Cl 3(aq) + Na. OH(aq) Fe(OH)3(s) + Na. Cl(aq) H 2 SO 4(aq) + Na. OH(aq) H 2 O(l) + Na 2 SO 4(aq) 6. KOH(aq) + Cu. SO 4(aq) 1. 2. 3. 4. K 2 SO 4(aq) + Cu(OH)2(s)

Mixed Practice • 1. 2. 3. 4. 5. State the type, predict the products, and balance the following reactions: Ba. Cl 2 + H 2 SO 4 Ba. SO 4 + HCl C 6 H 12 + O 2 CO 2 + H 2 O Zn + Cu. SO 4 Zn. SO 4 + Cu Cs + Br 2 Cs. Br Fe. CO 3 Fe. O + CO 2

Total Ionic Equations n n n Once you write the molecular equation (synthesis, decomposition, etc. ), you should check for reactants and products that are soluble or insoluble. We usually assume the reaction is in water We can use a solubility table to tell us what compounds dissolve in water. If the compound is soluble (does dissolve in water), then splits the compound into its component ions If the compound is insoluble (does NOT dissolve in water), then it remains as a compound

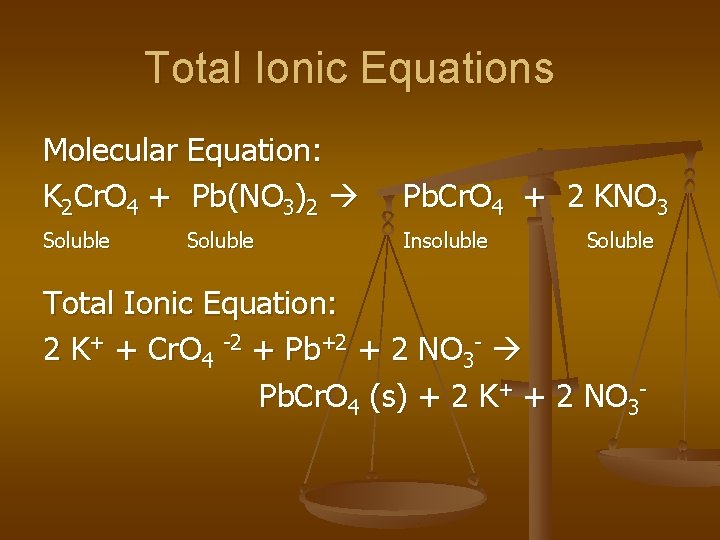
Total Ionic Equations Molecular Equation: K 2 Cr. O 4 + Pb(NO 3)2 Pb. Cr. O 4 + 2 KNO 3 Soluble Insoluble Soluble Total Ionic Equation: 2 K+ + Cr. O 4 -2 + Pb+2 + 2 NO 3 - Pb. Cr. O 4 (s) + 2 K+ + 2 NO 3 -

Net Ionic Equations These are the same as total ionic equations, but you should cancel out ions that appear on BOTH sides of the equation Total Ionic Equation: 2 K+ + Cr. O 4 -2 + Pb+2 + 2 NO 3 - Pb. Cr. O 4 (s) + 2 K+ + 2 NO 3 Net Ionic Equation: Cr. O 4 -2 + Pb+2 Pb. Cr. O 4 (s) n

Net Ionic Equations n Try this one! Write the molecular, total ionic, and net ionic equations for this reaction: Silver nitrate reacts with Lead (II) Chloride in hot water. Ag. NO 3 + Pb. Cl 2 Molecular: 2 Ag. NO 3 + Pb. Cl 2 2 Ag. Cl + Pb(NO 3)2 Total Ionic: 2 Ag+ + 2 NO 3 - + Pb+2 + 2 Cl- 2 Ag. Cl (s) + Pb+2 + 2 NO 3 Net Ionic: Ag+ + Cl- Ag. Cl (s)
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Antigentest åre
Antigentest åre Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Are kc and kp equal
Are kc and kp equal Types of chemical reactions redox
Types of chemical reactions redox Types of reactions
Types of reactions 4 types of chemical reactions
4 types of chemical reactions Reaction type
Reaction type 4 types of chemical reactions
4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions Five chemical change
Five chemical change 5 general types of chemical reactions
5 general types of chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions Types of reactions
Types of reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Three types of chemical reactions
Three types of chemical reactions Combustion reaction
Combustion reaction Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry How to write half reactions
How to write half reactions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Classify each polygon
Classify each polygon Proportional relationships in chemical reactions
Proportional relationships in chemical reactions Hcl and sodium hydrogen carbonate
Hcl and sodium hydrogen carbonate Predicting products of chemical reactions
Predicting products of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions Chapter 10 chemical reactions
Chapter 10 chemical reactions The calculations of quantities in chemical reactions
The calculations of quantities in chemical reactions Immunoelectrophoresis
Immunoelectrophoresis Predicting products of chemical reactions
Predicting products of chemical reactions Chemistry predicting products
Chemistry predicting products Section 3 predicting the products of chemical reactions
Section 3 predicting the products of chemical reactions Unit 11 chemical reactions
Unit 11 chemical reactions Toxic reactions chemical equations worksheet answers
Toxic reactions chemical equations worksheet answers What is the role of enzymes in chemical reactions
What is the role of enzymes in chemical reactions Describing chemical reactions
Describing chemical reactions