The Activity Series Predicting Single Displacement Reactions THE


- Slides: 6

The Activity Series Predicting Single Displacement Reactions

THE ACTIVITY SERIES • Not every single-replacement reaction will happen. • The element on the reactant side must be more “active” than the one it could replace. • Scientists have listed the order of activity of elements in THE ACTIVITY SERIES.

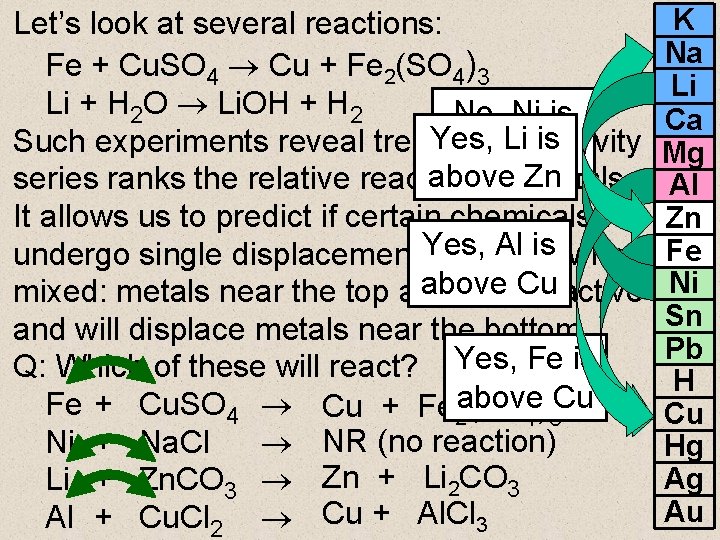
Let’s look at several reactions: Fe + Cu. SO 4 Cu + Fe 2(SO 4)3 Li + H 2 O Li. OH + H 2 No, Ni is Yes, Li is Such experiments reveal trends. The activity below Na above series ranks the relative reactivity of Zn metals. It allows us to predict if certain chemicals will Al is when undergo single displacement Yes, reactions above mixed: metals near the top are most. Cu reactive and will displace metals near the bottom. Q: Which of these will react? Yes, Fe is Fe + Cu. SO 4 Cu + Fe 2 above (SO 4)3 Cu Ni + Na. Cl NR (no reaction) Li + Zn. CO 3 Zn + Li 2 CO 3 Al + Cu. Cl 2 Cu + Al. Cl 3 K Na Li Ca Mg Al Zn Fe Ni Sn Pb H Cu Hg Ag Au

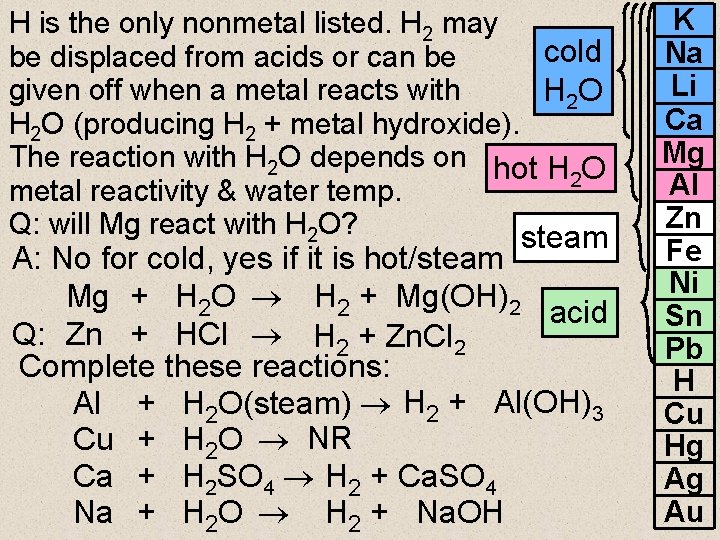
H is the only nonmetal listed. H 2 may cold be displaced from acids or can be given off when a metal reacts with H 2 O (producing H 2 + metal hydroxide). The reaction with H 2 O depends on hot H O 2 metal reactivity & water temp. Q: will Mg react with H 2 O? steam A: No for cold, yes if it is hot/steam Mg + H 2 O H 2 + Mg(OH)2 acid Q: Zn + HCl H 2 + Zn. Cl 2 Complete these reactions: Al + H 2 O(steam) H 2 + Al(OH)3 Cu + H 2 O NR Ca + H 2 SO 4 H 2 + Ca. SO 4 Na + H 2 O H 2 + Na. OH K Na Li Ca Mg Al Zn Fe Ni Sn Pb H Cu Hg Ag Au

Other Activity Series Information • All metals will have a specific place in the activity series. For simplicity, only the most common metals are shown. • The metals near the top of the activity series are more reactive because their valence electrons are more easily removed. • On tests and exams the activity series may appear as K, Na, … Ag, Au; you must remember that K is reactive, Au is not. • If the valence of a metal is not indicated in the question, use its most common valence (in bold on your periodic table) to determine the correct chemical formula.

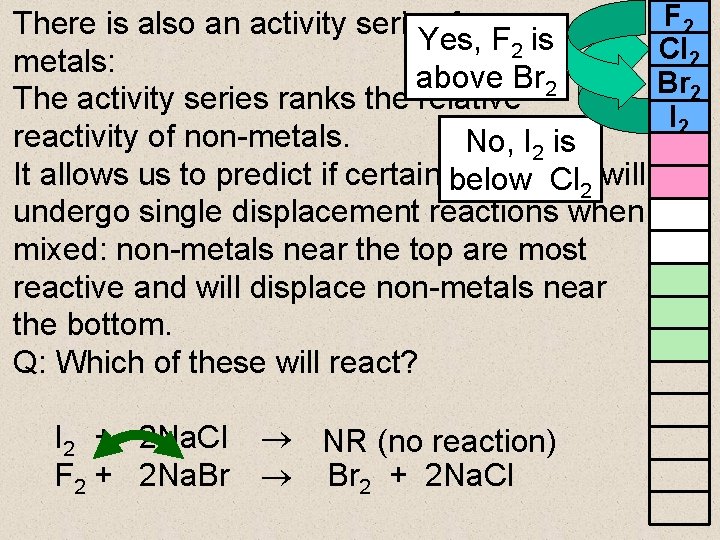
There is also an activity series for non. Yes, F 2 is metals: above Br 2 The activity series ranks the relative reactivity of non-metals. No, I 2 is It allows us to predict if certain below chemicals Cl 2 will undergo single displacement reactions when mixed: non-metals near the top are most reactive and will displace non-metals near the bottom. Q: Which of these will react? I 2 + 2 Na. Cl NR (no reaction) F 2 + 2 Na. Br 2 + 2 Na. Cl F 2 Cl 2 Br 2 I 2