Unit G Mathematics of Chemical Reactions Gravimetric Stoichiometry





- Slides: 8

Unit G Mathematics of Chemical Reactions (Gravimetric Stoichiometry)

G. 1 Introduction: Mathematics of Chemical Reactions n n This unit studies the use of mathematics in balanced equations to determine the amount of reactant needed or predict the amount of product that will be formed when the reaction is run. Gravimetric: involves the use of a balance Ø Ø n Gravi-: using gravity (mass) -metric: to measure Stoichiometry (based on two Greek words) Ø Ø Stoicheion: “of the elements” -metry: to measure

G. 1 Introduction: Mathematics of Chemical Reactions n Stoichiometry is a review: Ø Ø Must understand the formulas of elements and ions (Unit B) Must be able to write chemical names and formulas (Unit D) Must be able to write and balance chemical equations (Unit E) Must be able to convert mass to moles and moles to mass (Unit F).

G. 1 Introduction: Mathematics of Chemical Reactions n Practical Importance of Stoichiometry 1. 2. 3. The gasoline-to-air mixture in a car. The proper proportions of ingredients in recipes. Antacid tablets to neutralize stomach acid may be harmful in excess.

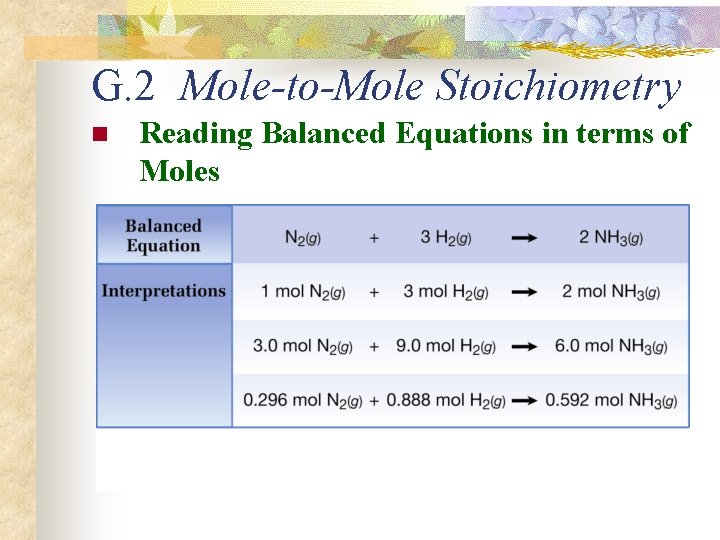
G. 2 Mole-to-Mole Stoichiometry n Reading Balanced Equations in terms of Moles

G. 2 Mole-to-Mole Stoichiometry n Generalized Solving Method 1. 2. 3. 4. Write the balanced chemical equation. List given amount under appropriate substance. Put a (? ) with the necessary units under the substance you are looking for. Multiply the given amount by the mole ratio: Round final answer using appropriate rules.

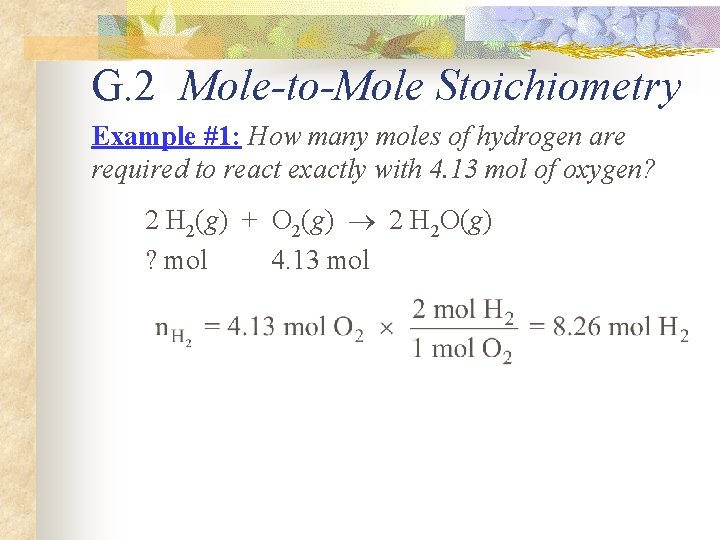
G. 2 Mole-to-Mole Stoichiometry Example #1: How many moles of hydrogen are required to react exactly with 4. 13 mol of oxygen? 2 H 2(g) + O 2(g) 2 H 2 O(g) ? mol 4. 13 mol

G. 2 Mole-to-Mole Stoichiometry Example #2: Determine the number of moles of natural gas (methane) that must undergo combustion to produce 1. 62 mol of water vapor. CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) ? mol 1. 62 mol
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Stoichiometry map for chemical reactions
Stoichiometry map for chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Gravimetric stoichiometry
Gravimetric stoichiometry Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Section 1 chemical changes
Section 1 chemical changes















