Reaction Stoichiometry Reaction Stoichiometry Process of using a












- Slides: 28

Reaction Stoichiometry

Reaction Stoichiometry Process of using a chemical equation to calculate relative masses of reactants and products

Info given by a chemical equation. CO + 2 H 2 CH 3 OH o Relative amounts can be describe with moles n n 1 mole CO reacts with 2 moles of H 2 produces 1 mole of CH 3 OH

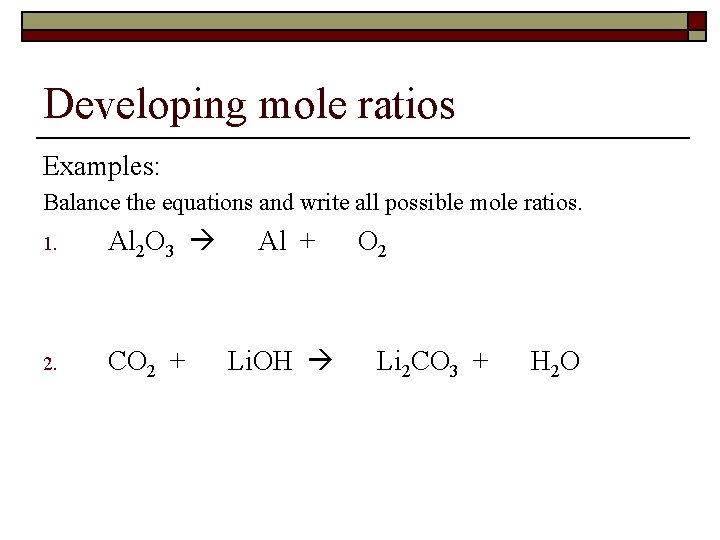
Developing mole ratios Mole Ratio – conversion factor obtained from a balanced chemical reaction CAN ONLY USE MOLES TO RELATE AMOUNTS IN A REACTION!!! o Conversion factors relating moles of any two substances involved in a reaction. o Obtained from the balanced chemical equation.

Developing mole ratios Examples: Balance the equations and write all possible mole ratios. 1. Al 2 O 3 2. CO 2 + Al + Li. OH O 2 Li 2 CO 3 + H 2 O

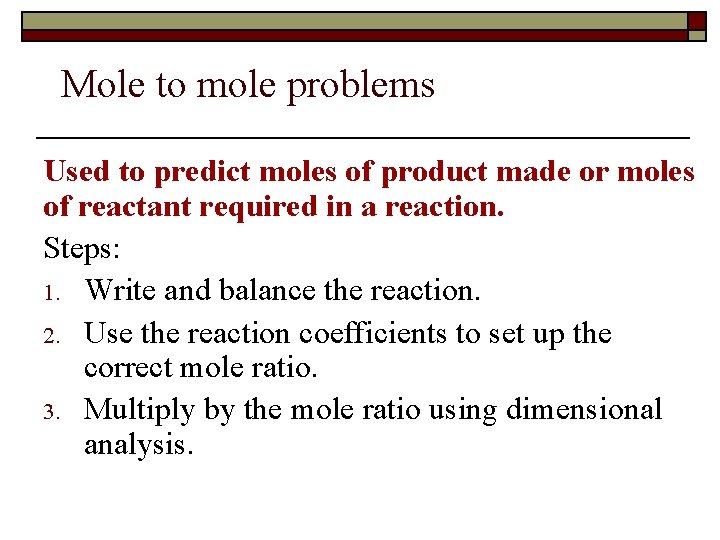
Mole to mole problems Used to predict moles of product made or moles of reactant required in a reaction. Steps: 1. Write and balance the reaction. 2. Use the reaction coefficients to set up the correct mole ratio. 3. Multiply by the mole ratio using dimensional analysis.

Mole to mole problems Examples: 1. What number of moles of O 2 will be produced by the decomposition of 5. 8 moles of water?

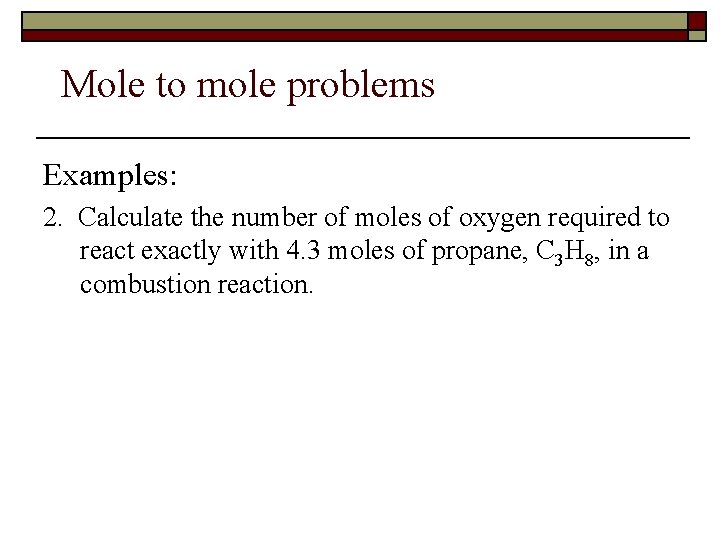
Mole to mole problems Examples: 2. Calculate the number of moles of oxygen required to react exactly with 4. 3 moles of propane, C 3 H 8, in a combustion reaction.

Mole to mole problems Examples: 3. Ammonia is used in huge quantities as a fertilizer. It is manufactured by combining nitrogen and hydrogen. Calculate the number of moles of NH 3 that can be made from 1. 30 moles H 2 with excess N 2.


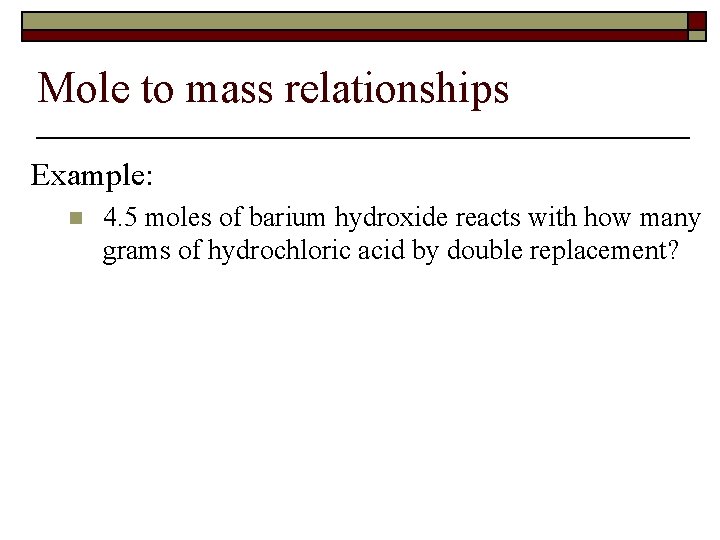
Mole to mass relationships Example: n 4. 5 moles of barium hydroxide reacts with how many grams of hydrochloric acid by double replacement?

Mass to mole relationships Practice n Sodium phosphate reacts with chromium III chloride. If 34. 3 grams of sodium chloride is produced, how many moles of chromium III phosphate are produced?

Mole to mass relationships Practice: n n 3. 25 moles of zinc reacts with excess hydrochloric acid by single replacement. How many grams of hydrogen gas are produced? Ammonium iodide and calcium nitrate react by double replacement. If 15. 4 moles of ammonium iodide is used, how many grams of each product are produced?

Mass to mole relationships Example n In photosynthesis, plants use energy from the sun to produce glucose, C 6 H 12 O 6, and O 2 from the reaction of CO 2 and H 2 O. How many moles of glucose are produced when 65. 8 grams of carbon dioxide is reacted with excess water?

Mass to mole relationships Practice n n How many moles of oxygen are produce in the photosynthesis reaction if 35. 0 grams of water are reacted with excess carbon dioxide? Sodium phosphate reacts with chromium III chloride. If 34. 3 grams of sodium chloride is produced, how many moles of chromium III phosphate are produced?


Mass to mass problems Used to predict grams of product made or grams of reactant required in a reaction. Steps: 1. Write and balance the reaction. 2. Convert the grams provided into moles using molar mass. 3. Use the reaction coefficients to set up the correct mole ratio. 4. Multiply by the mole ratio using dimensional analysis. 5. Convert the moles found into grams using molar mass.

Mass to mass problems Examples: 1. Consider the reaction of powdered aluminum metal and finely ground iodine to produce aluminum iodide. Calculate the mass of I 2 needed to just react with 35. 0 grams of aluminum?

Mass to mass problems Examples: 2. Solid lithium hydroxide is used in space vehicles to remove exhaled carbon dioxide from the living environment. The products are solid lithium carbonate and liquid water. What mass of gaseous carbon dioxide can 1. 00 x 103 grams of lithium hydroxide absorb?



Ideal stoichiometric calculations o Ideal conditions n n n All reactants and products are 100% pure. All reactants are converted to products. Very few reactions actually are run under ideal conditions o o o Side reactions occur Ideal pressure or temperature difficult to obtain Impurities/contaminants

Yield Vocabulary Theoretical yield – amount of product predicted by the balanced chemical equation mole ratio Actual yield – actual amount of product produced in a chemical reaction Why are the different? 1. Side reactions 2. Bond energy of reactants may be lower (endothermic)

Yield Formula Percent Yield = o o actual yield x 100 theoretical yield High percent yield is desired. Important when manufacturing chemicals

Limiting Reactant Vocabulary Limiting reactant – present in short supply relative to the others; completely used in the reaction Excess reactant – more present than can be used in the reaction Stoichiometric mixture - the ratio of the reactants equals the chemical equation’s mole ratio for the reactants

Limiting Reactant problems Used to determine which reactant will be consumed (run out) first. Steps: 1. Write and balance the reaction. 2. Convert the grams provided for each reactant into moles using molar mass. 3. Divide the number of moles of each reactant by the reaction coefficient of that reactant. The smallest quotient is the limiting reactant.

Limiting Reactant problems Example 1. Suppose 25 g of nitrogen gas and 5. 0 g of hydrogen gas are mixed and reacted to form ammonia, NH 3. n Identify the limiting reactant n Calculate the mass of ammonia which should have been produced if the reaction has 100% yield. n Determine which reactant is in excess and how much reactant remains. When the two gases were mixed 20. g of ammonia were actually produced. n Calculate the percent yield of the reaction.

Limiting Reactants Examples 2. 6. 85 g of aluminum reacts with 6. 85 g of sulfuric acid by single replacement. An experiment showed that 8. 00 g of aluminum sulfate were produced. n n n Identify the limiting reactant Calculate the mass of aluminum sulfate which should have been produced. Calculate the percent yield of the reaction.