STOICHIOMETRY What is stoichiometry l Stoichiometry is the













- Slides: 21

STOICHIOMETRY

What is stoichiometry? l. Stoichiometry is the quantitative study of reactants and products in a chemical reaction.

What You Should Expect l l Given : Amount of reactants Question: how much of products can be formed. l Example l l 2 A + 2 B 3 C Given 20. 0 grams of A and sufficient B, how many grams of C can be produced?

What do you need? i. iii. iv. You will need to use molar ratios, molar masses, balancing and interpreting equations, and conversions between grams and moles. Note: This type of problem is often called "mass-mass. "

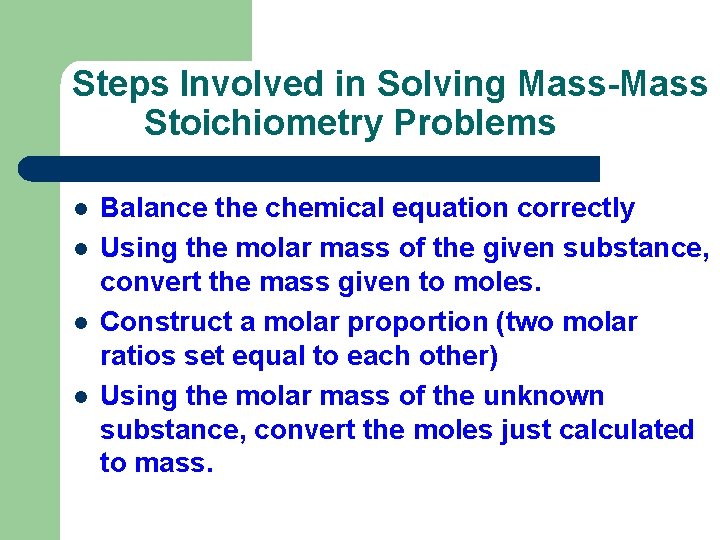
Steps Involved in Solving Mass-Mass Stoichiometry Problems l l Balance the chemical equation correctly Using the molar mass of the given substance, convert the mass given to moles. Construct a molar proportion (two molar ratios set equal to each other) Using the molar mass of the unknown substance, convert the moles just calculated to mass.

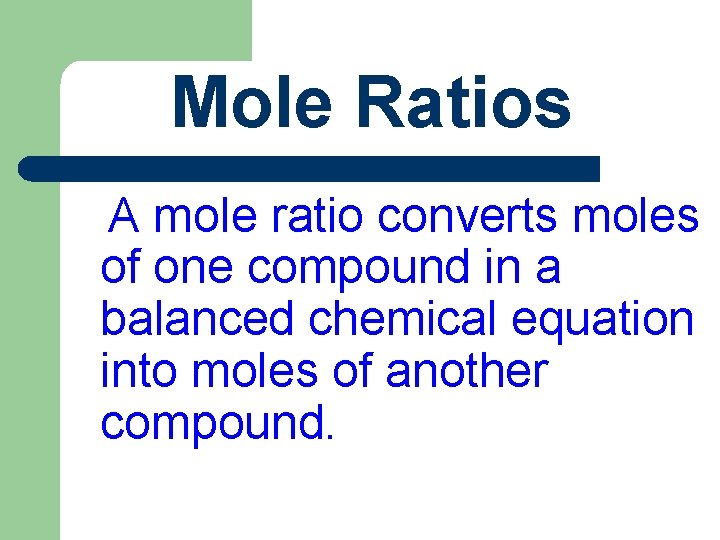
Mole Ratios A mole ratio converts moles of one compound in a balanced chemical equation into moles of another compound.

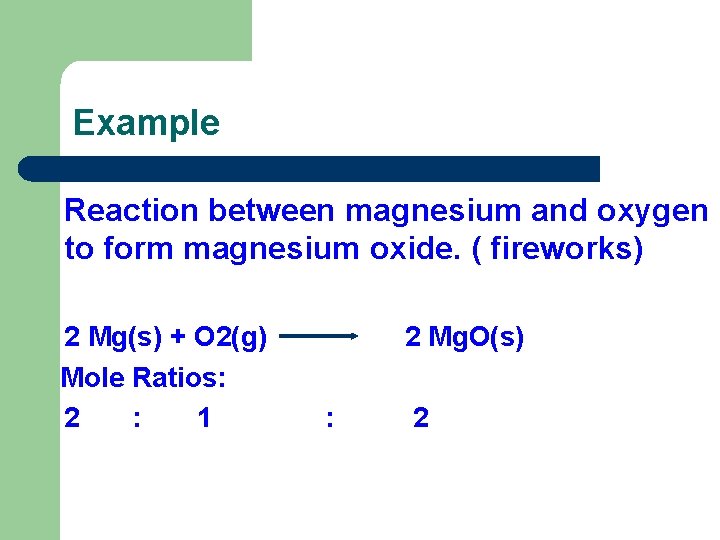
Example Reaction between magnesium and oxygen to form magnesium oxide. ( fireworks) 2 Mg(s) + O 2(g) Mole Ratios: 2 : 1 2 Mg. O(s) : 2

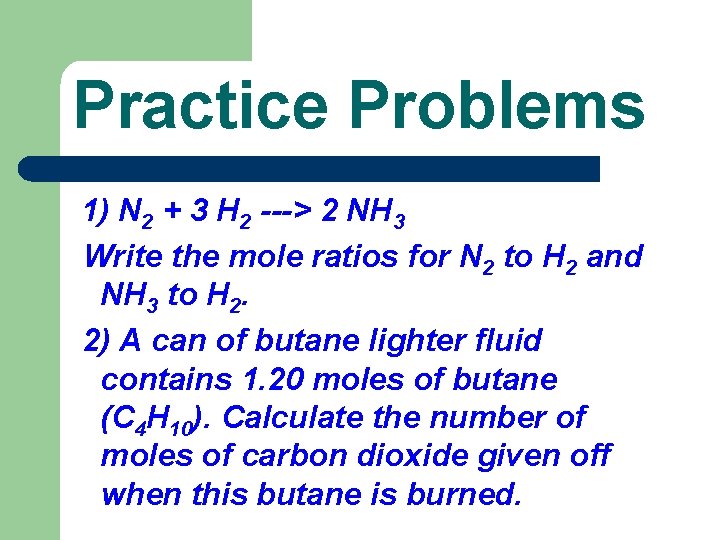
Practice Problems 1) N 2 + 3 H 2 ---> 2 NH 3 Write the mole ratios for N 2 to H 2 and NH 3 to H 2. 2) A can of butane lighter fluid contains 1. 20 moles of butane (C 4 H 10). Calculate the number of moles of carbon dioxide given off when this butane is burned.

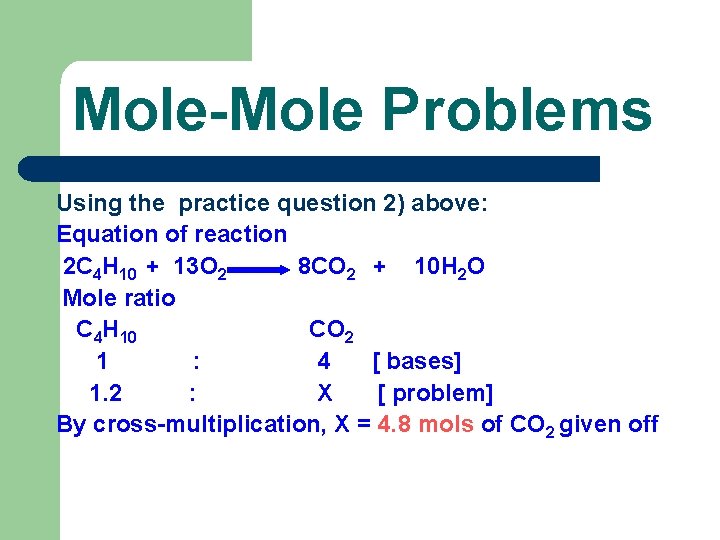
Mole-Mole Problems Using the practice question 2) above: Equation of reaction 2 C 4 H 10 + 13 O 2 8 CO 2 + 10 H 2 O Mole ratio C 4 H 10 CO 2 1 : 4 [ bases] 1. 2 : X [ problem] By cross-multiplication, X = 4. 8 mols of CO 2 given off

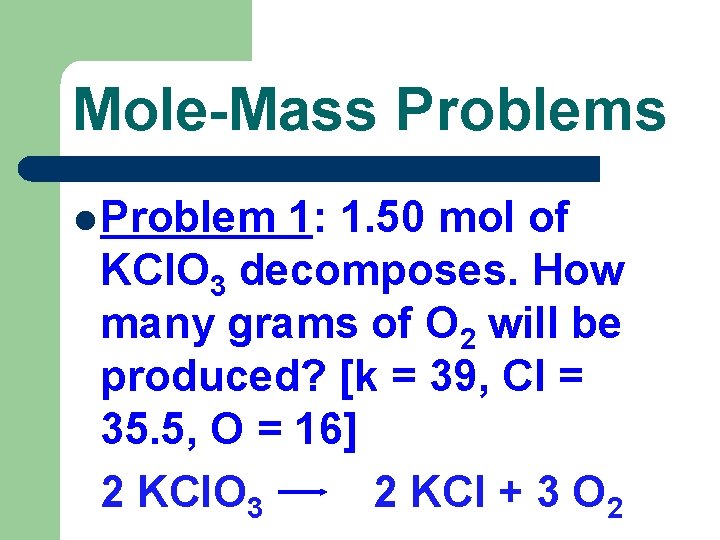
Mole-Mass Problems l Problem 1: 1. 50 mol of KCl. O 3 decomposes. How many grams of O 2 will be produced? [k = 39, Cl = 35. 5, O = 16] 2 KCl. O 3 2 KCl + 3 O 2

Three steps…Get Your Correct Answer Use mole ratio l Get the answer in moles and then l Convert to Mass. [Simple Arithmetic] l Hello! If you are given a mass in the problem, you will need to convert this to moles first. Ok?

Let’s go! 2 KCl. O 3 2 : 1. 50 : X = 2. 25 mol Convert to mass 2 KCl + 3 O 2 3 X 2. 25 mol x 32. 0 g/mol = 72. 0 grams Cool!

Try This: We want to produce 2. 75 mol of KCl. How many grams of KCl. O 3 would be required? Soln l KCl. O 3 : KCl 2 : 2 X : 2. 75 X = 2. 75 mol In mass: 2. 75 mol X 122. 55 g/mol = 337 grams zooo zimple!

Mass-Mass Problems l l There are four steps involved in solving these problems: Make sure you are working with a properly balanced equation. Convert grams of the substance given in the problem to moles. Construct two ratios - one from the problem and one from the equation and set them equal. Solve for "x, " which is usually found in the ratio from the problem. Convert moles of the substance just solved for into grams.

Mass-Volume Problems Just follow mass problem to the penultimate level

Like this: There are four steps involved in solving these problems: l Make sure you are working with a properly balanced equation. l Convert grams of the substance given in the problem to moles. l Construct two ratios - one from the problem and one from the equation and set them equal. Solve for "x, " which is usually found in the ratio from the problem. l Convert moles of the substance just solved for into Volume.

Conversion of mole to volume No of moles = Volume Molar volume Can you remember a similar equation?

Molar volume The molar volume is the volume occupied by one mole of ideal gas at STP. Its value is: 22. 4 dm 3

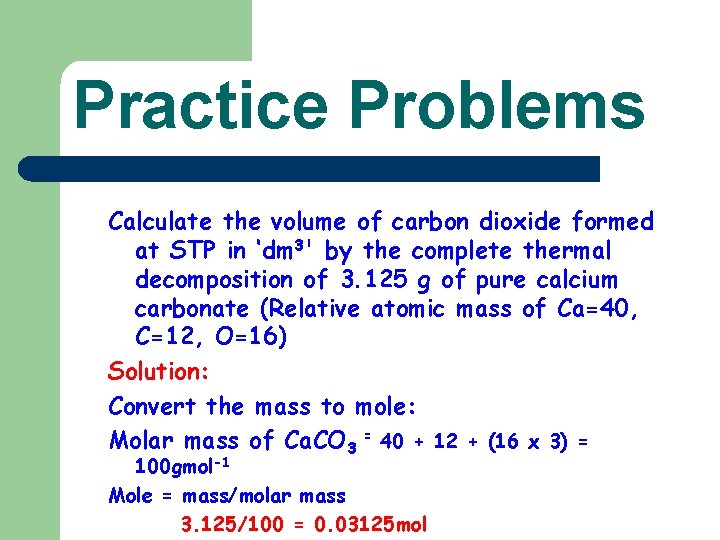
Practice Problems Calculate the volume of carbon dioxide formed at STP in ‘dm 3' by the complete thermal decomposition of 3. 125 g of pure calcium carbonate (Relative atomic mass of Ca=40, C=12, O=16) Solution: Convert the mass to mole: Molar mass of Ca. CO 3 = 40 + 12 + (16 x 3) = 100 gmol-1 Mole = mass/molar mass 3. 125/100 = 0. 03125 mol

Practice Problems As per the equation, Mole ratio 1 : 1 problem 0. 03125 mol X X = 0. 03125 mol of CO 2 Convert mole to volume [slide 17] 3 x 22. 4)dm Volume = (0. 03125 = 0. 7 dm 3

This powerpoint was kindly donated to www. worldofteaching. com http: //www. worldofteaching. com is home to over a thousand powerpoints submitted by teachers. This is a completely free site and requires no registration. Please visit and I hope it will help in your teaching.
 Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Frameset trong html5
Frameset trong html5 Sơ đồ cơ thể người
Sơ đồ cơ thể người Số.nguyên tố
Số.nguyên tố Tư thế ngồi viết
Tư thế ngồi viết đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Tư thế worm breton là gì
Tư thế worm breton là gì ưu thế lai là gì
ưu thế lai là gì Thẻ vin
Thẻ vin Cái miệng nó xinh thế chỉ nói điều hay thôi
Cái miệng nó xinh thế chỉ nói điều hay thôi Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Bổ thể
Bổ thể Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Tư thế ngồi viết
Tư thế ngồi viết V cc cc
V cc cc Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Alleluia hat len nguoi oi
Alleluia hat len nguoi oi Hươu thường đẻ mỗi lứa mấy con
Hươu thường đẻ mỗi lứa mấy con Diễn thế sinh thái là
Diễn thế sinh thái là