STOICHIOMETRY What is stoichiometry l Stoichiometry is the










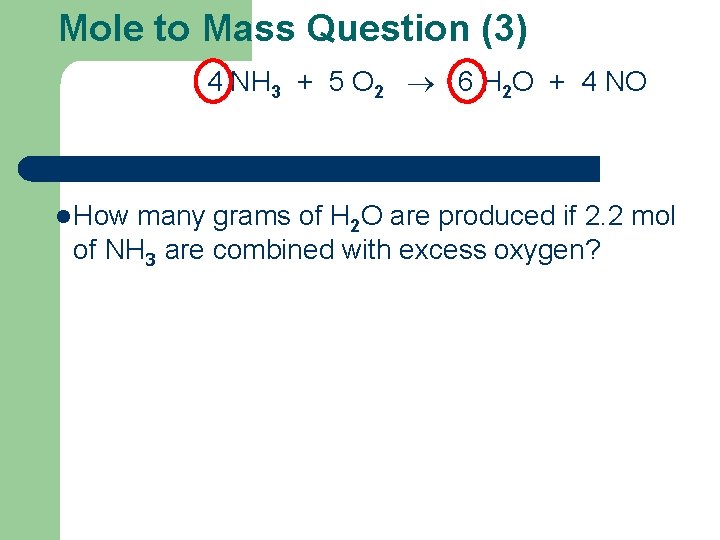
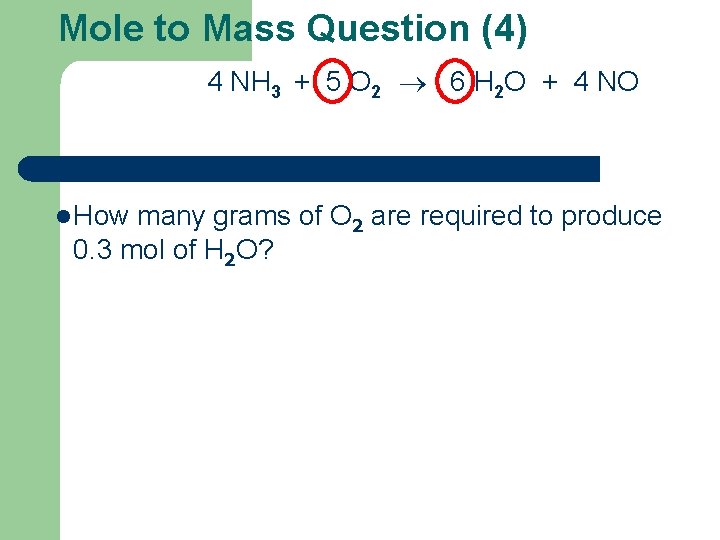


- Slides: 29

STOICHIOMETRY

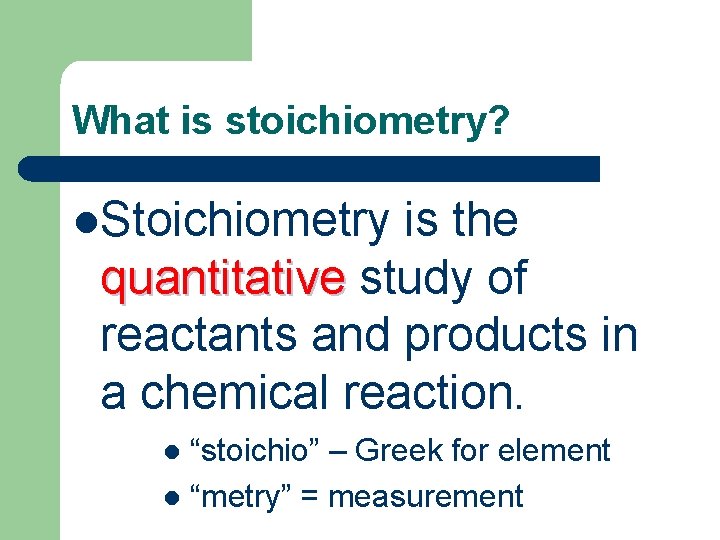
What is stoichiometry? l. Stoichiometry is the quantitative study of quantitative reactants and products in a chemical reaction. “stoichio” – Greek for element l “metry” = measurement l

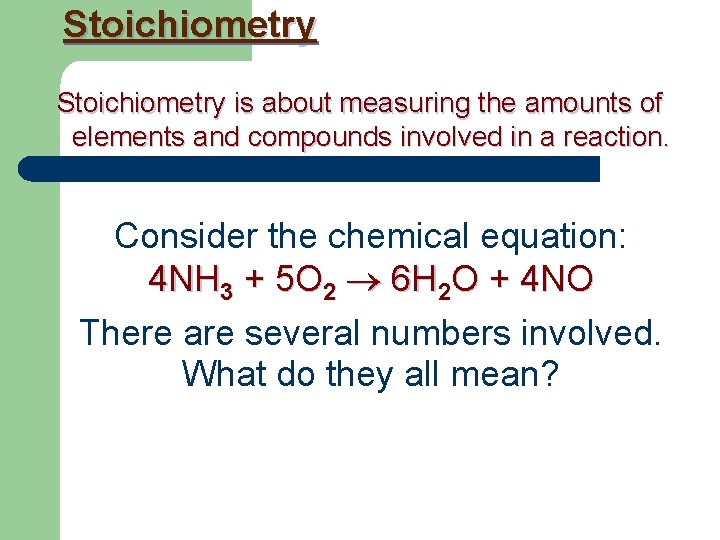
Stoichiometry is about measuring the amounts of elements and compounds involved in a reaction. Consider the chemical equation: 4 NH 3 + 5 O 2 6 H 2 O + 4 NO There are several numbers involved. What do they all mean?

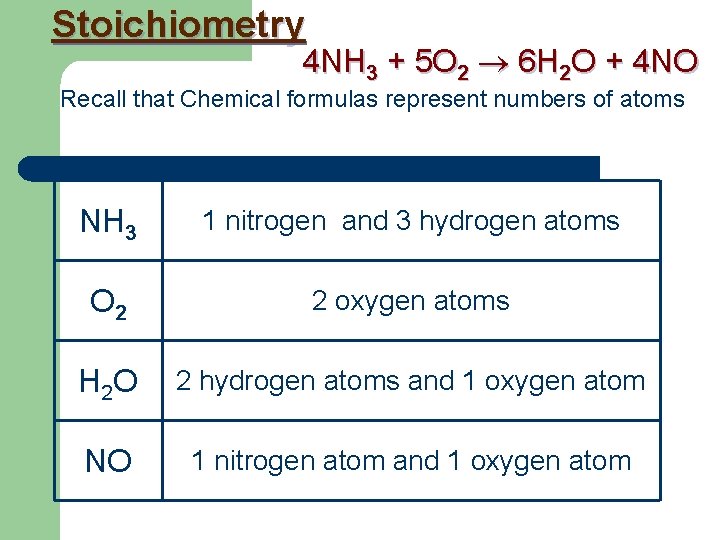
Stoichiometry 4 NH 3 + 5 O 2 6 H 2 O + 4 NO Recall that Chemical formulas represent numbers of atoms NH 3 1 nitrogen and 3 hydrogen atoms O 2 2 oxygen atoms H 2 O 2 hydrogen atoms and 1 oxygen atom NO 1 nitrogen atom and 1 oxygen atom

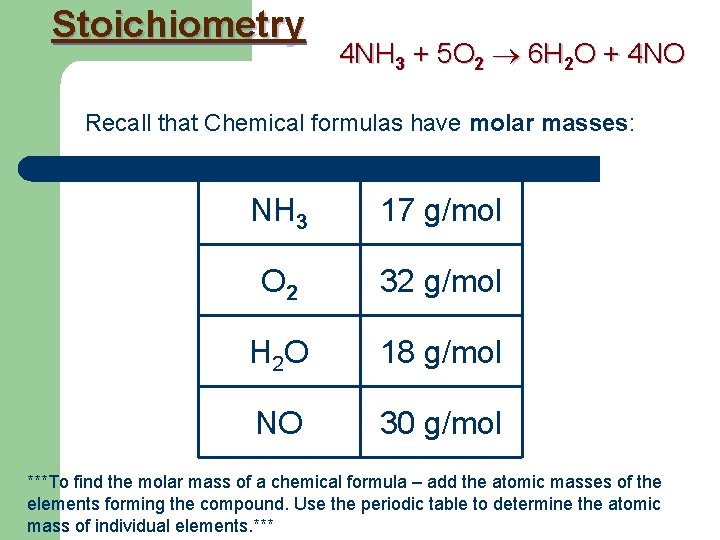
Stoichiometry 4 NH 3 + 5 O 2 6 H 2 O + 4 NO Recall that Chemical formulas have molar masses: NH 3 17 g/mol O 2 32 g/mol H 2 O 18 g/mol NO 30 g/mol ***To find the molar mass of a chemical formula – add the atomic masses of the elements forming the compound. Use the periodic table to determine the atomic mass of individual elements. ***

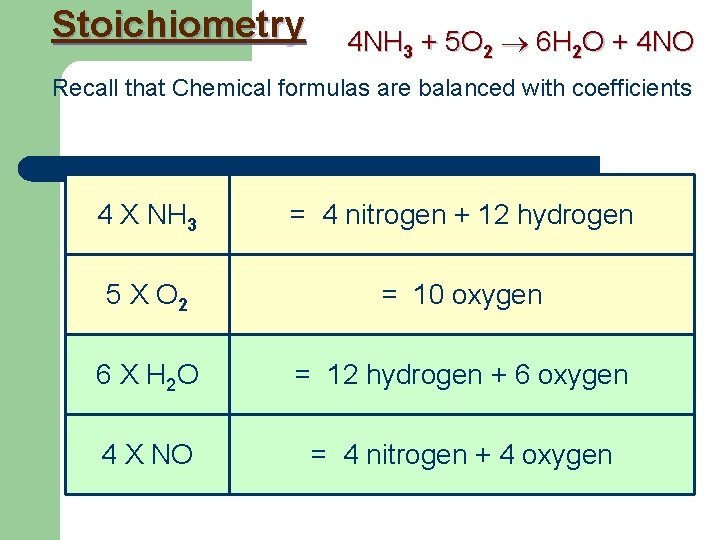
Stoichiometry 4 NH 3 + 5 O 2 6 H 2 O + 4 NO Recall that Chemical formulas are balanced with coefficients 4 X NH 3 = 4 nitrogen + 12 hydrogen 5 X O 2 = 10 oxygen 6 X H 2 O = 12 hydrogen + 6 oxygen 4 X NO = 4 nitrogen + 4 oxygen

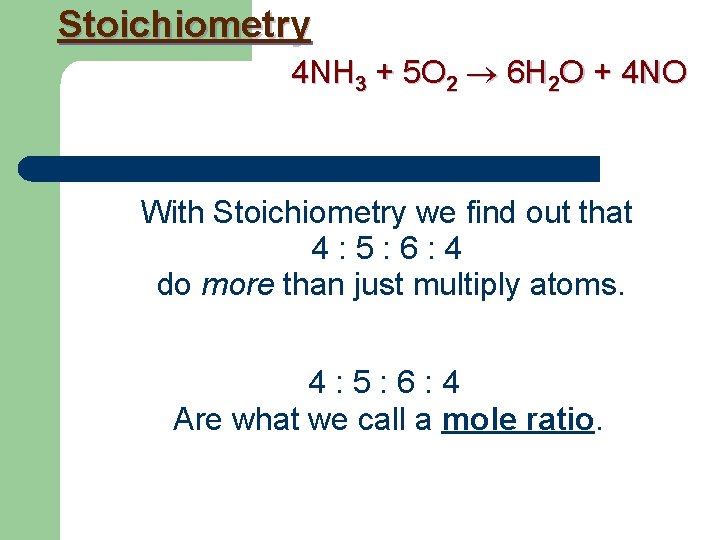
Stoichiometry 4 NH 3 + 5 O 2 6 H 2 O + 4 NO With Stoichiometry we find out that 4 : 5 : 6 : 4 do more than just multiply atoms. 4 : 5 : 6 : 4 Are what we call a mole ratio.

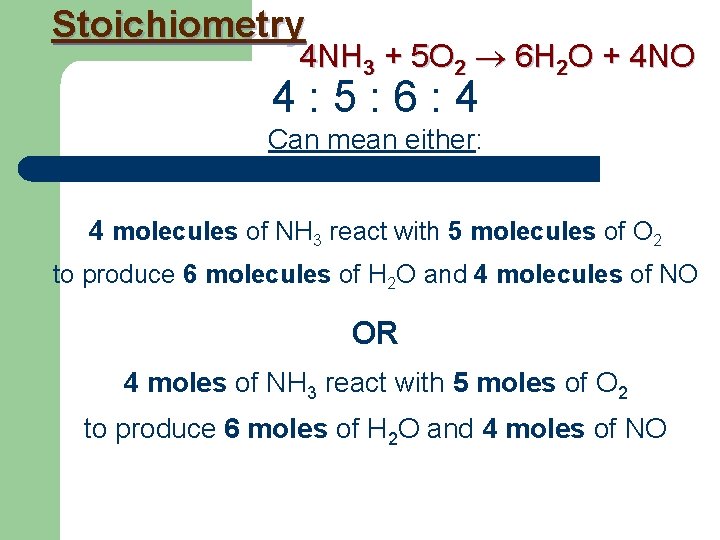
Stoichiometry 4 NH 3 + 5 O 2 6 H 2 O + 4 NO 4 : 5 : 6 : 4 Can mean either: 4 molecules of NH 3 react with 5 molecules of O 2 to produce 6 molecules of H 2 O and 4 molecules of NO OR 4 moles of NH 3 react with 5 moles of O 2 to produce 6 moles of H 2 O and 4 moles of NO

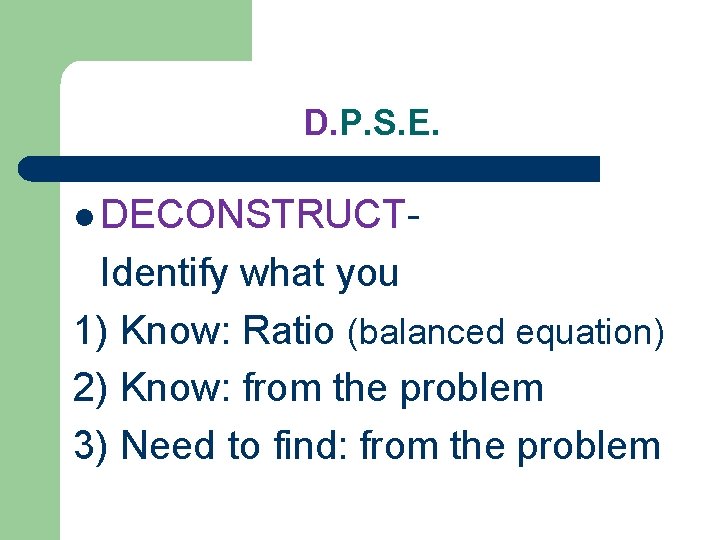
D. P. S. E. l DECONSTRUCT- Identify what you 1) Know: Ratio (balanced equation) 2) Know: from the problem 3) Need to find: from the problem

D. P. S. E. l Plan- Decide what type of pathway or equation you need to use. l We are going to start with using ratios, equalities, cross-multiplying and then dividing as needed (as we did last semester)

D. P. S. E. l Solve – Insert numbers with units in proper places in your Plan

D. P. S. E. l Evaluate – Circle your answer with units, and check it to see if it makes sense. l Is it too big, too small, does it make logical sense?

What You Should Expect l l Given : Amount of reactants Question: how much of the products can be formed. l Example l l 2 A + 2 B 3 C Given 20. 0 grams of A and sufficient B, how many grams of C can be produced?

What do you need? i. iii. iv. You will need to use molar ratios, molar masses, balancing and interpreting equations, and conversions between grams and moles.

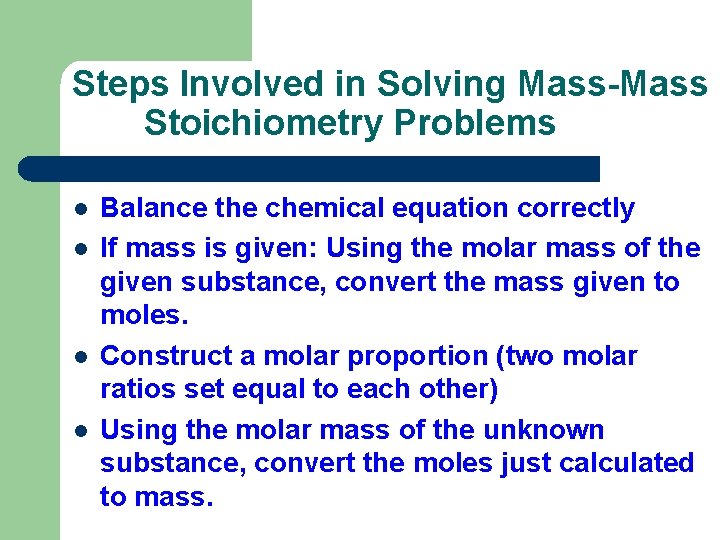
Steps Involved in Solving Mass-Mass Stoichiometry Problems l l Balance the chemical equation correctly If mass is given: Using the molar mass of the given substance, convert the mass given to moles. Construct a molar proportion (two molar ratios set equal to each other) Using the molar mass of the unknown substance, convert the moles just calculated to mass.

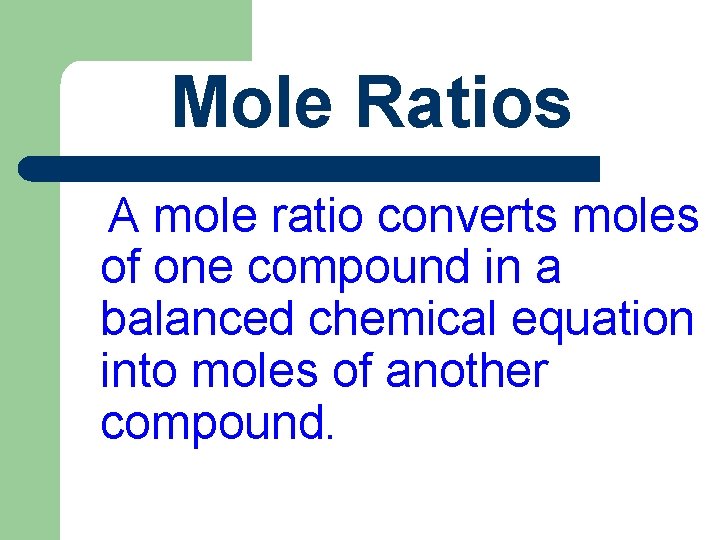
Mole Ratios A mole ratio converts moles of one compound in a balanced chemical equation into moles of another compound.

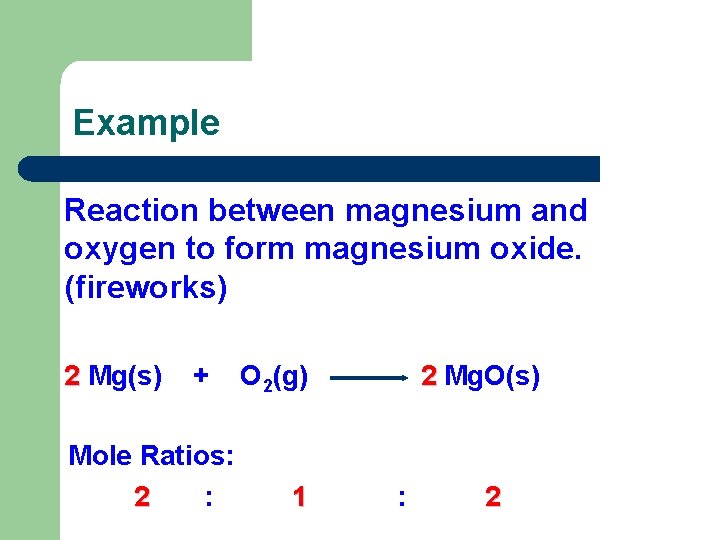
Example Reaction between magnesium and oxygen to form magnesium oxide. (fireworks) 2 Mg(s) + Mole Ratios: 2 : O 2(g) 1 2 Mg. O(s) : 2

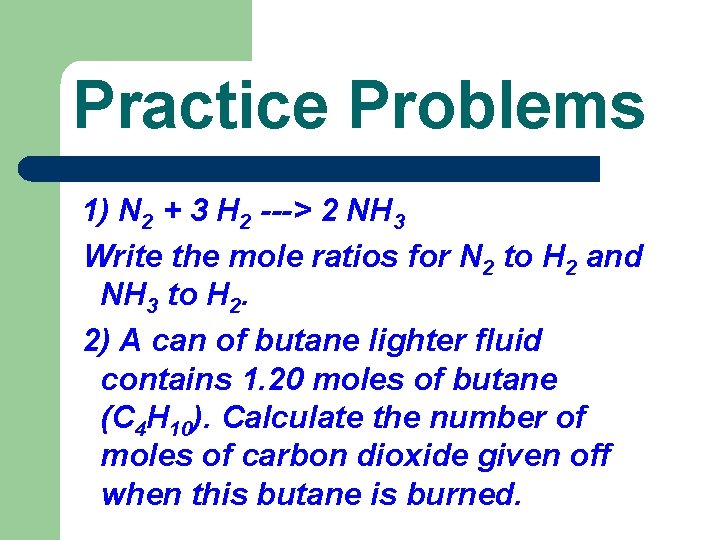
Practice Problems 1) N 2 + 3 H 2 ---> 2 NH 3 Write the mole ratios for N 2 to H 2 and NH 3 to H 2. 2) A can of butane lighter fluid contains 1. 20 moles of butane (C 4 H 10). Calculate the number of moles of carbon dioxide given off when this butane is burned.

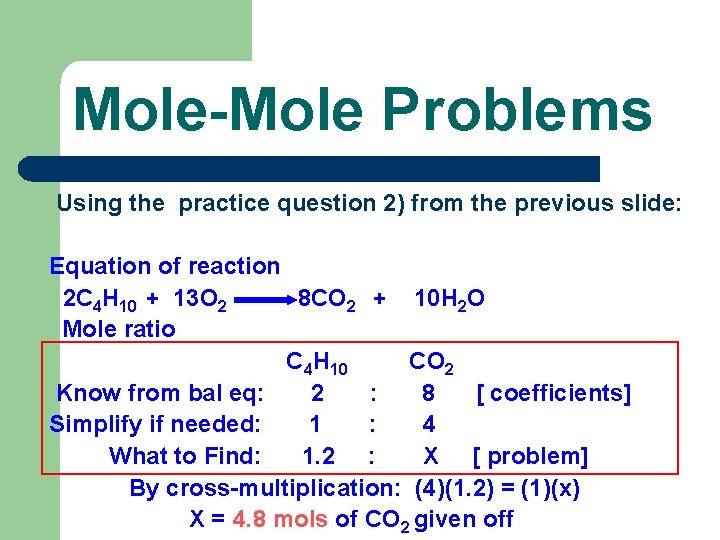
Mole-Mole Problems Using the practice question 2) from the previous slide: Equation of reaction 2 C 4 H 10 + 13 O 2 8 CO 2 + 10 H 2 O Mole ratio C 4 H 10 CO 2 Know from bal eq: 2 : 8 [ coefficients] Simplify if needed: 1 : 4 What to Find: 1. 2 : X [ problem] By cross-multiplication: (4)(1. 2) = (1)(x) X = 4. 8 mols of CO 2 given off

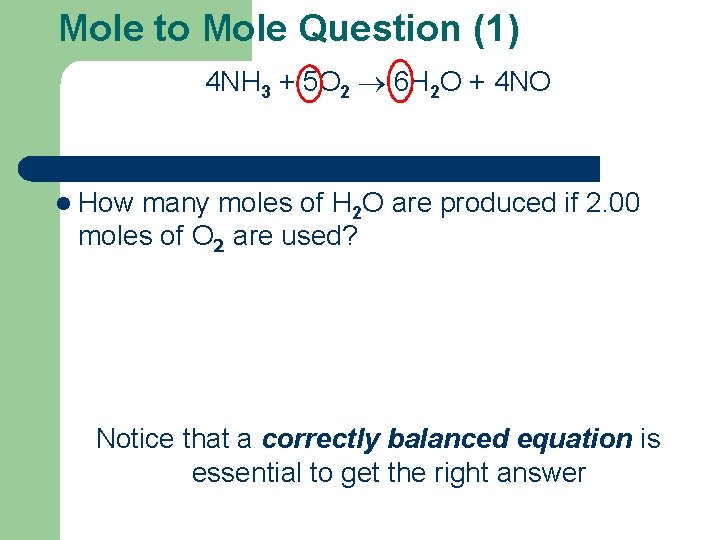
Mole to Mole Question (1) 4 NH 3 + 5 O 2 6 H 2 O + 4 NO l How many moles of H 2 O are produced if 2. 00 moles of O 2 are used? Notice that a correctly balanced equation is essential to get the right answer

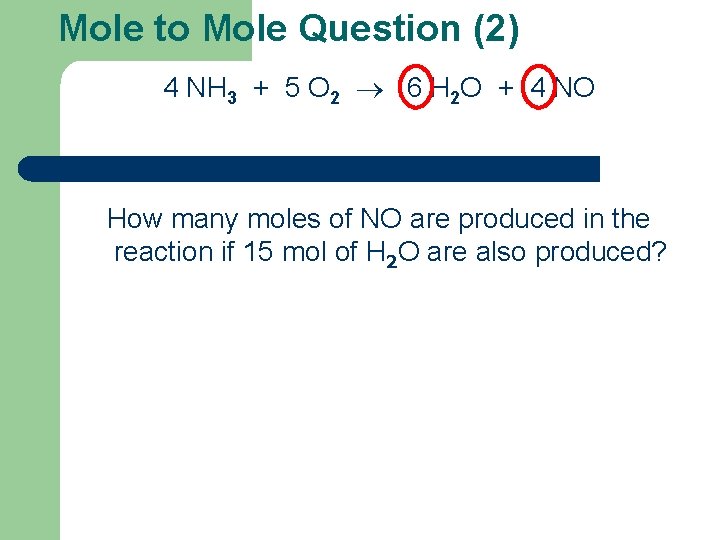
Mole to Mole Question (2) 4 NH 3 + 5 O 2 6 H 2 O + 4 NO How many moles of NO are produced in the reaction if 15 mol of H 2 O are also produced?

Mole-Mass Problems Make sure you have molar masses worked out for all compounds l Set up for grid making sure to use units correctly l Cross-multiply and isolate your variable (be sure to recognize those units that cancel out) l

Example for Mole-Mass Problems l Problem 1: 1. 50 mol of KCl. O 3 decomposes. How many grams of O 2 will be produced? [K = 39, Cl = 35. 5, O = 16] 2 KCl. O 3 2 KCl + 3 O 2

Let’s go! Molar masses: 245. 10 1. 50 mol of KCl. O 3 decomposes. How many grams of O 2 will be produced? 149. 10 96 2 KCl. O 3 2 KCl + 3 O 2 2 mol : 96 g 1. 50 mol : X g (96 g)(1. 50 mol)=(2 mol)(X g) 144 g/mol = (2 mol) (X g) 144 g/mol / 2 mol = 72 g O 2

Try This: We want to produce 2. 75 mol of KCl. How many grams of KCl. O 3 would be required? Soln l KCl. O 3 KCl : 245. 10 g : 2 mol X g : 2. 75 mol (2 mol)(X g) = (2. 75 mol)(245. 10 g) (2 mol)(X g) = 674. 025 g/mol X = 337. 01 grams of KCl. O 3
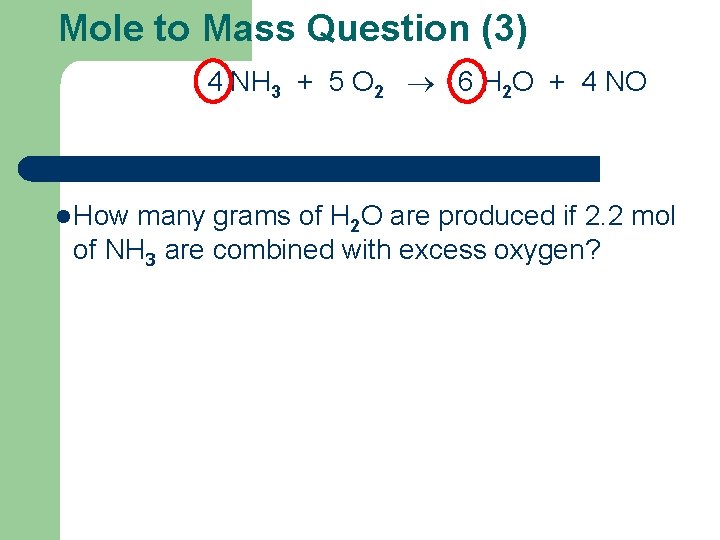
Mole to Mass Question (3) 4 NH 3 + 5 O 2 6 H 2 O + 4 NO l How many grams of H 2 O are produced if 2. 2 mol of NH 3 are combined with excess oxygen?
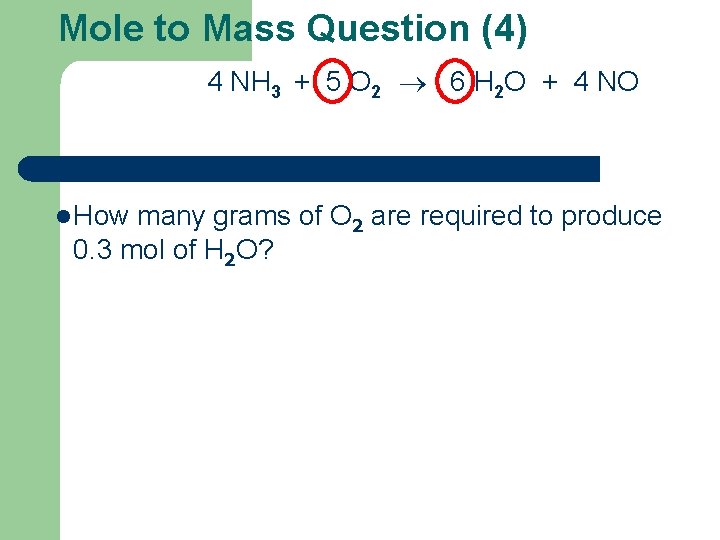
Mole to Mass Question (4) 4 NH 3 + 5 O 2 6 H 2 O + 4 NO l How many grams of O 2 are required to produce 0. 3 mol of H 2 O?

Mass to Mass Question (5) 4 NH 3 + 5 O 2 6 H 2 O + 4 NO l How many grams of NO is produced if 12 g of O 2 is combined with excess ammonia?

Have we learned it yet? Try these on your own - 4 NH 3 + 5 O 2 6 H 2 O + 4 NO a) How many moles of H 2 O can be made using 1. 6 mol NH 3? b) What mass of NH 3 is needed to make 0. 75 mol NO? c) How many grams of NO can be made from 47 g of NH 3?
 Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Fecboak
Fecboak Một số thể thơ truyền thống
Một số thể thơ truyền thống Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Frameset trong html5
Frameset trong html5 Hệ hô hấp
Hệ hô hấp Các số nguyên tố là gì
Các số nguyên tố là gì Tư thế ngồi viết
Tư thế ngồi viết đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Tư thế worm breton
Tư thế worm breton ưu thế lai là gì
ưu thế lai là gì Thẻ vin
Thẻ vin Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Bổ thể
Bổ thể Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Tư thế ngồi viết
Tư thế ngồi viết V cc cc
V cc cc Thể thơ truyền thống
Thể thơ truyền thống Phép trừ bù
Phép trừ bù Hát lên người ơi
Hát lên người ơi Hổ đẻ mỗi lứa mấy con
Hổ đẻ mỗi lứa mấy con Diễn thế sinh thái là
Diễn thế sinh thái là đại từ thay thế
đại từ thay thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Công thức tiính động năng
Công thức tiính động năng Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em