Stoichiometry What is Stoichiometry Stoichiometry is a mathematical









- Slides: 19

Stoichiometry

What is Stoichiometry? Stoichiometry is a mathematical area of chemistry that relates quantities of reactants and products in a chemical reaction.

Why is chemical stoichiometry important? Stoichiometric calculations increase efficiency in laboratories, decrease waste in manufacturing, and produce products more quickly. So what does that mean for you and me? Cheaper products!! It all comes down to the all mighty dollar!

Recipe Analogy A BLT sandwich equation… If you have 9 bacon slices (Ba), how many BLT sandwiches (Ba. Le. T) can you make? If you need to make 20 Ba. Le. T, how much lettuce (Le) do you need? If you have 4 slices of bread (Bd) how many slices of tomato (T) do you need?

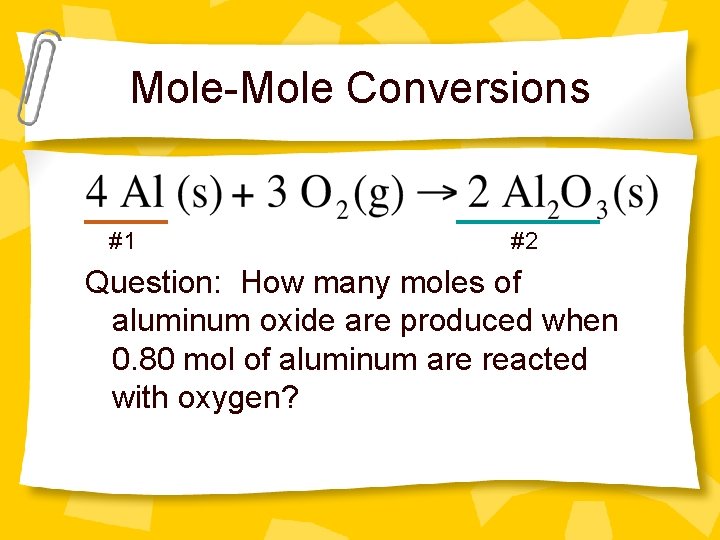
Mole-Mole Conversions #1 #2 Question: How many moles of aluminum oxide are produced when 0. 80 mol of aluminum are reacted with oxygen?

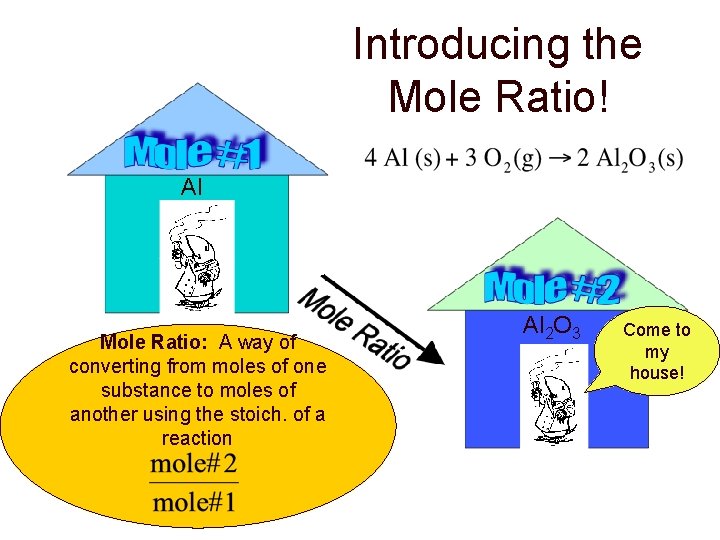
Introducing the Mole Ratio! Al Mole Ratio: A way of converting from moles of one substance to moles of another using the stoich. of a reaction Al 2 O 3 Come to my house!

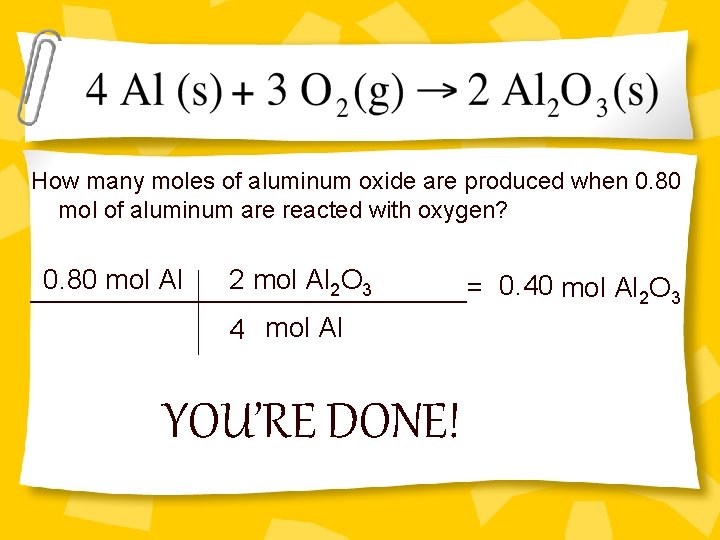
How many moles of aluminum oxide are produced when 0. 80 mol of aluminum are reacted with oxygen? 0. 80 mol Al 2 O 3 0. 40 mol Al 2 O 3 ______________= 4 mol Al YOU’RE DONE!

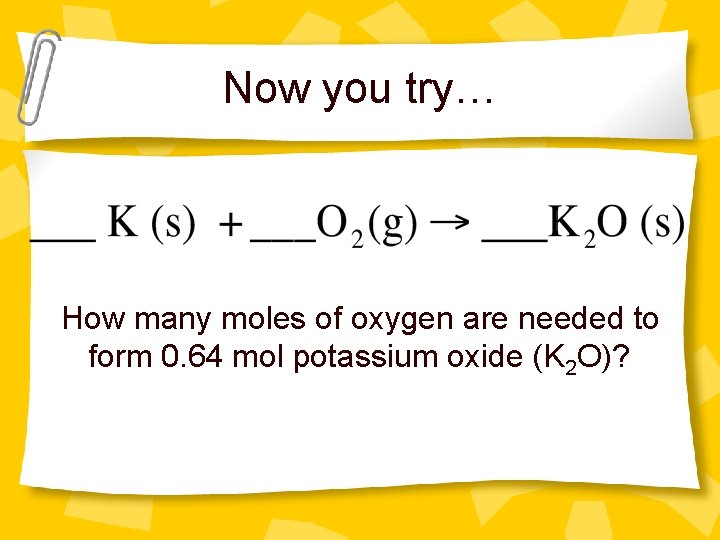
Now you try… How many moles of oxygen are needed to form 0. 64 mol potassium oxide (K 2 O)?

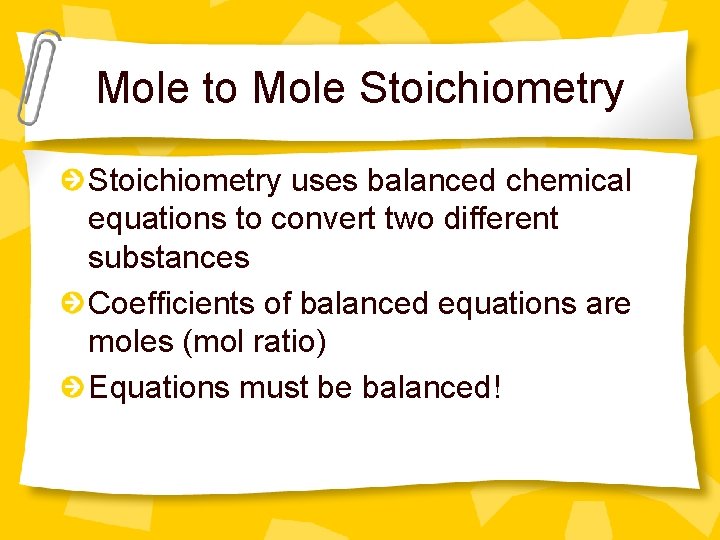
Mole to Mole Stoichiometry uses balanced chemical equations to convert two different substances Coefficients of balanced equations are moles (mol ratio) Equations must be balanced!

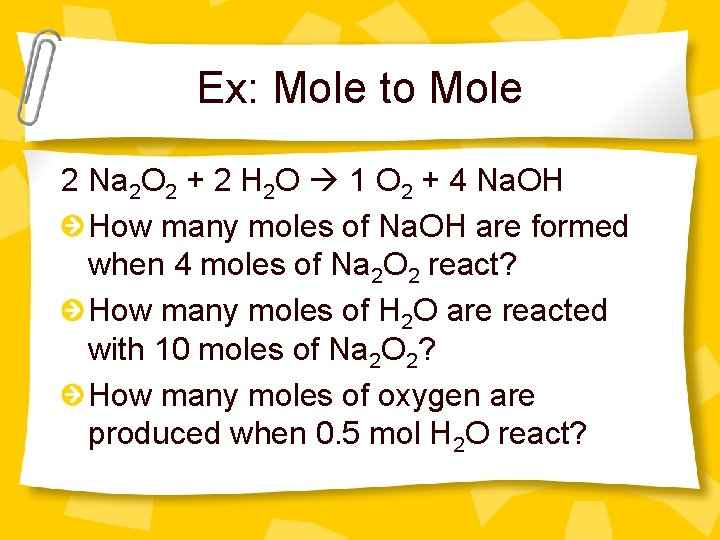
Ex: Mole to Mole 2 Na 2 O 2 + 2 H 2 O 1 O 2 + 4 Na. OH How many moles of Na. OH are formed when 4 moles of Na 2 O 2 react? How many moles of H 2 O are reacted with 10 moles of Na 2 O 2? How many moles of oxygen are produced when 0. 5 mol H 2 O react?

Mass to mole Molar mass: the mass of an element contained in 1 mole of that element. Use the molar mass of the element off of the periodic table and add “g” to the end. —don’t forget to round—

Exp: (Left side) – 70 g of chlorine equals how many moles of chlorine? – How many grams are in 5 moles of carbon?

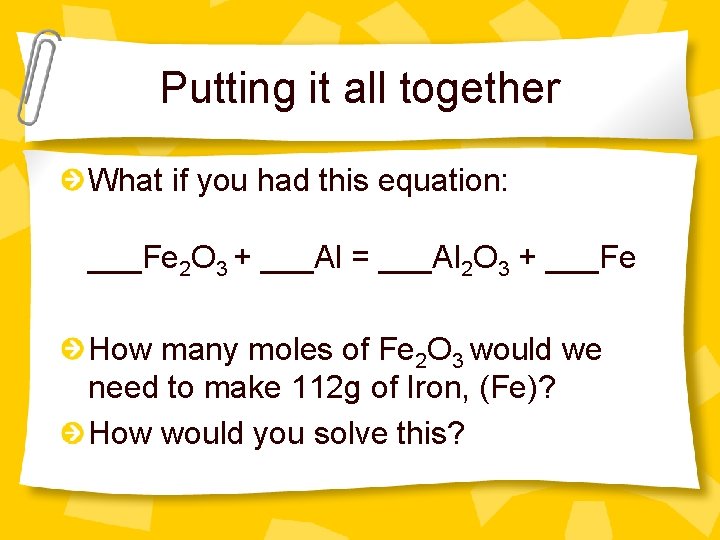
Putting it all together What if you had this equation: ___Fe 2 O 3 + ___Al = ___Al 2 O 3 + ___Fe How many moles of Fe 2 O 3 would we need to make 112 g of Iron, (Fe)? How would you solve this?

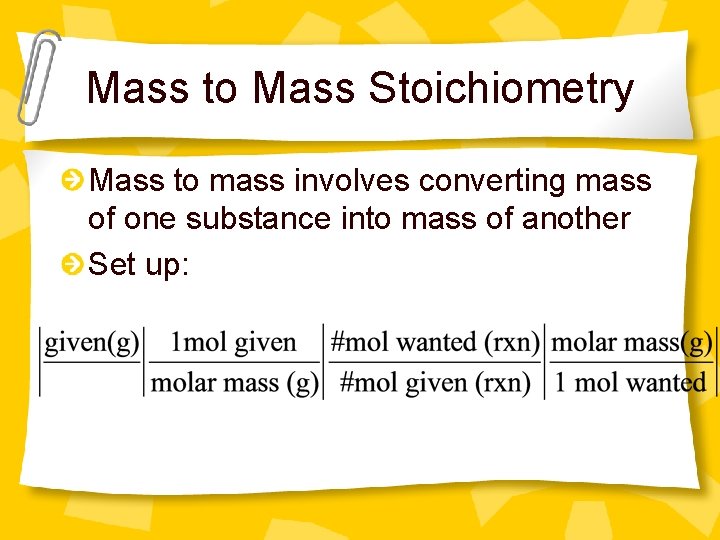
Mass to Mass Stoichiometry Mass to mass involves converting mass of one substance into mass of another Set up:

Example Mass to Mass 5 C + 2 SO 2 1 CS 2 + 4 CO How many grams CS 2 form when 60 g carbon react? How many grams of CO form from reacting 128 g SO 2?

Volume to Volume Stoich Can convert moles into liters Must have L, not m. L For gases at STP (standard temperature and pressure), one mole occupies a volume of 22. 4 L 1 mole

Example #1 What volume will 2. 00 moles of H 2 occupy at STP? #2 What volume will 48. 0 g of O 2 occupy at STP?

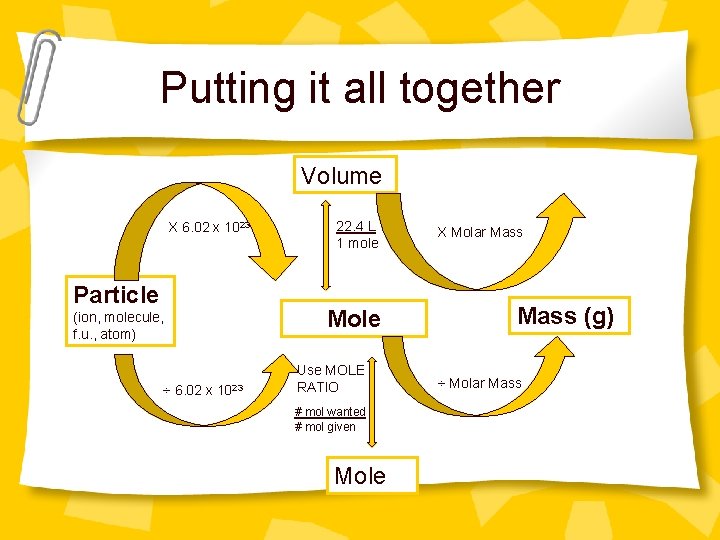
Putting it all together Volume X 6. 02 x 1023 Particle (ion, molecule, f. u. , atom) ÷ 6. 02 x 1023 22. 4 L 1 mole Mole Use MOLE RATIO # mol wanted # mol given Mole X Molar Mass (g) ÷ Molar Mass

How many boxes do I use and when do I use them?