Solids Liquids Gases Chapter 7 The Nature of










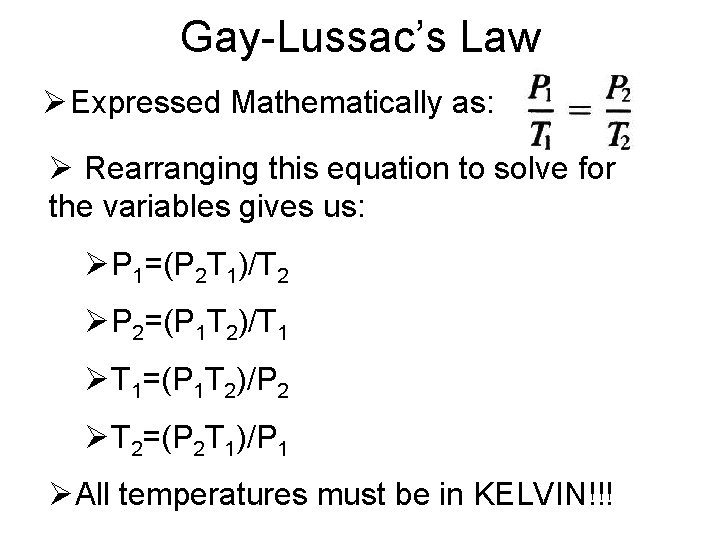


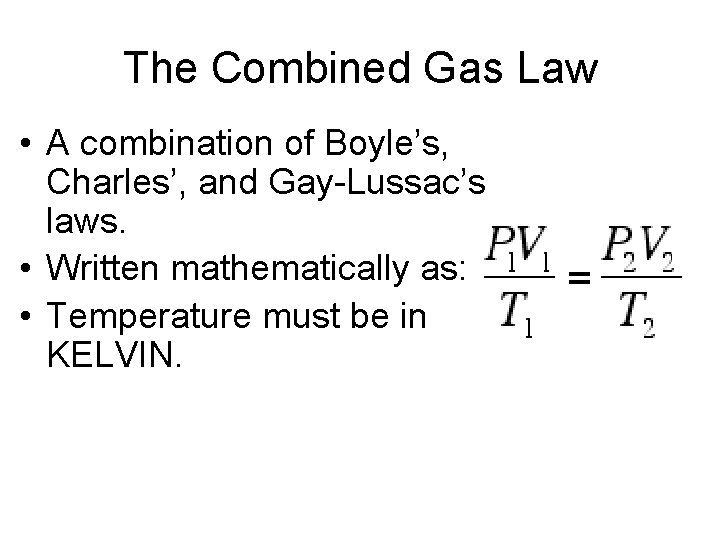



















- Slides: 68

Solids, Liquids, & Gases Chapter 7

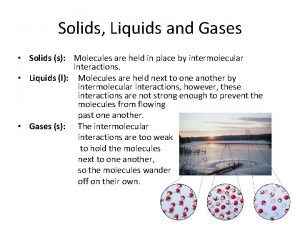
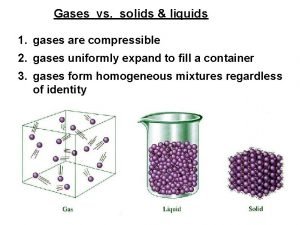
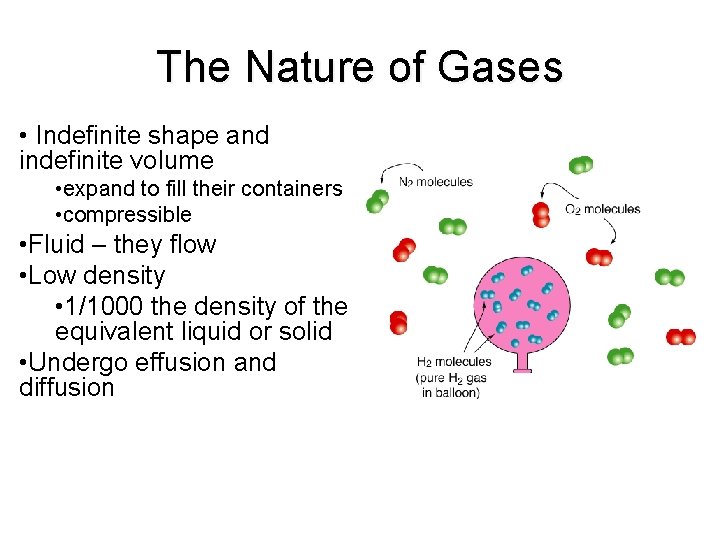
The Nature of Gases • Indefinite shape and indefinite volume • expand to fill their containers • compressible • Fluid – they flow • Low density • 1/1000 the density of the equivalent liquid or solid • Undergo effusion and diffusion

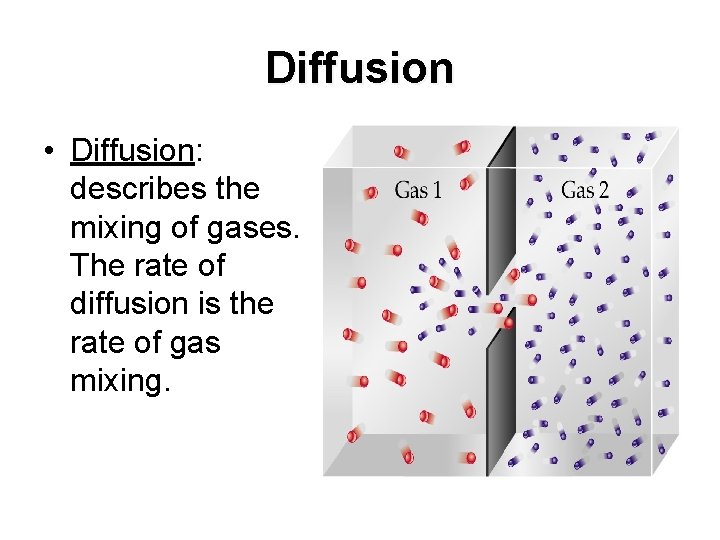
Diffusion • Diffusion: describes the mixing of gases. The rate of diffusion is the rate of gas mixing.

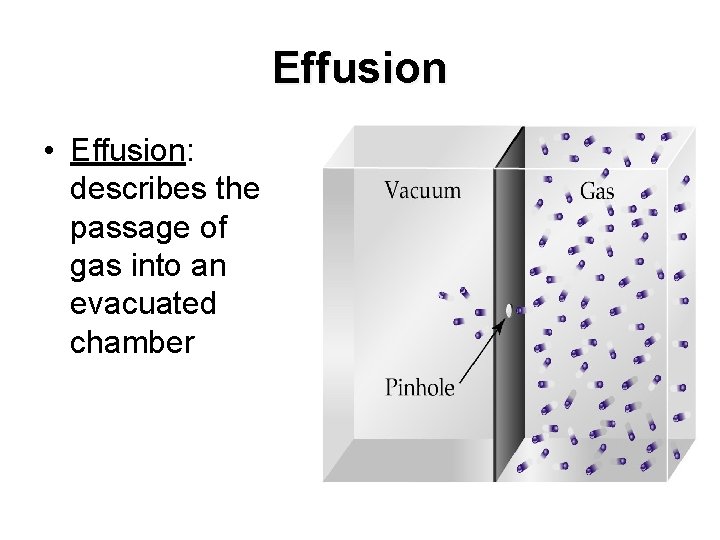
Effusion • Effusion: describes the passage of gas into an evacuated chamber

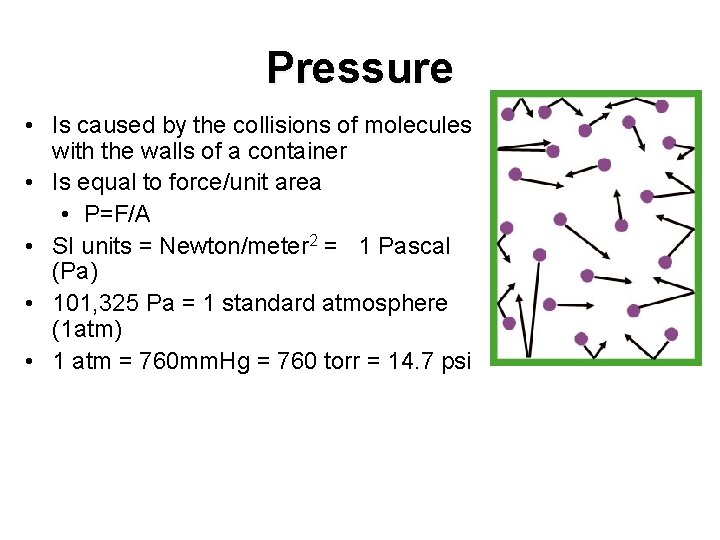
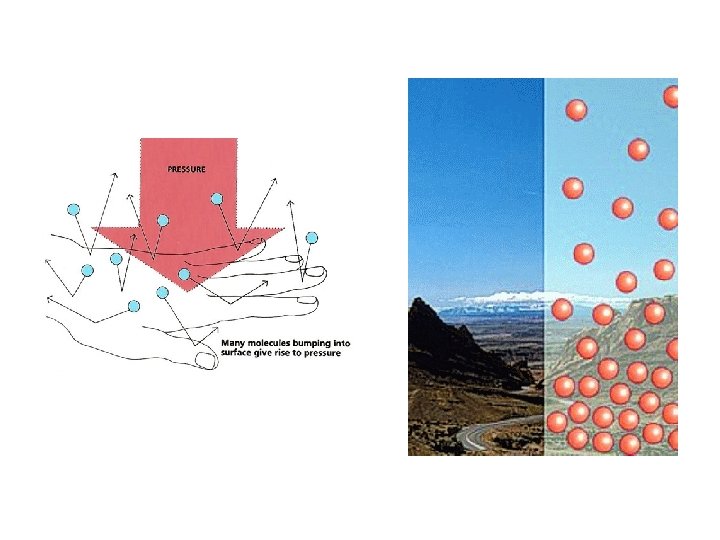
Pressure • Is caused by the collisions of molecules with the walls of a container • Is equal to force/unit area • P=F/A • SI units = Newton/meter 2 = 1 Pascal (Pa) • 101, 325 Pa = 1 standard atmosphere (1 atm) • 1 atm = 760 mm. Hg = 760 torr = 14. 7 psi



GAS LAWS Boyle’s Law Charles’ Law Gay-Lussac’s Law Avogadro’s. Law Ideal Gas Law Daltons Law

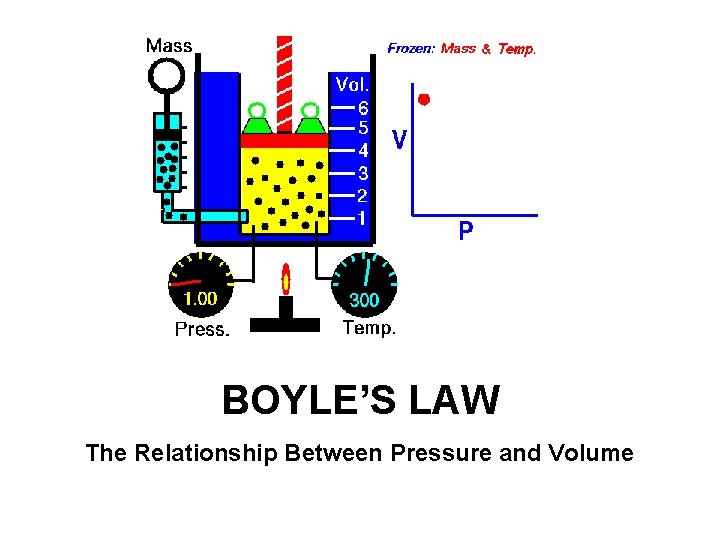
BOYLE’S LAW The Relationship Between Pressure and Volume

Robert Boyle (1627 -1691) Ø Boyle was born into an aristocratic Irish family Ø Became interested in medicine and the new science of Galileo and studied chemistry. Ø A founder and an influential fellow of the Royal Society of London Ø Wrote prolifically on science, philosophy, and theology.

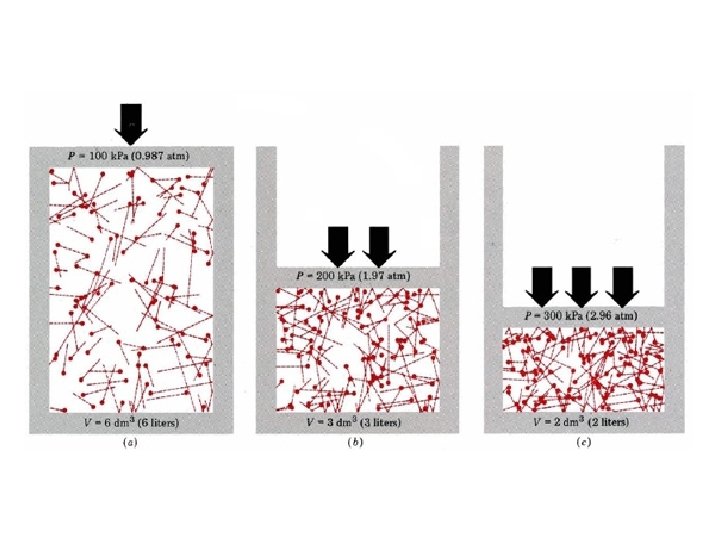
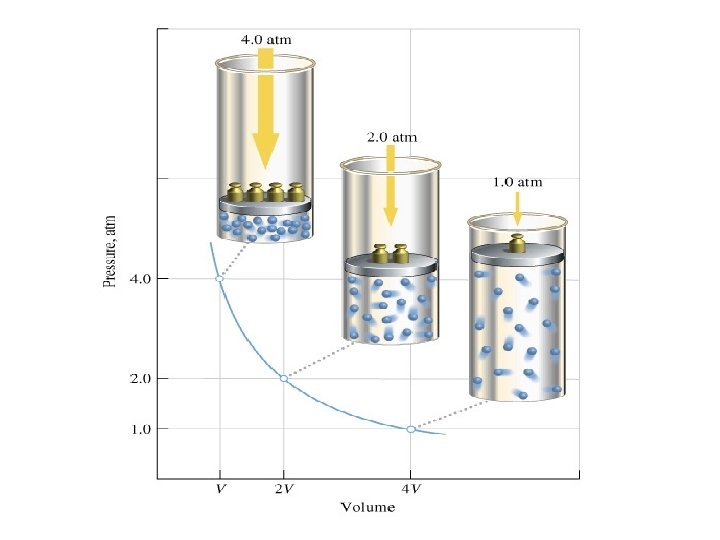
Boyle’s Law • Boyle’s Law: The volume of a gas is inversely proportional to the pressure applied to the gas when the temperature is kept constant. • Decrease in volume = Increase in pressure. • Increase in volume = Decrease the pressure


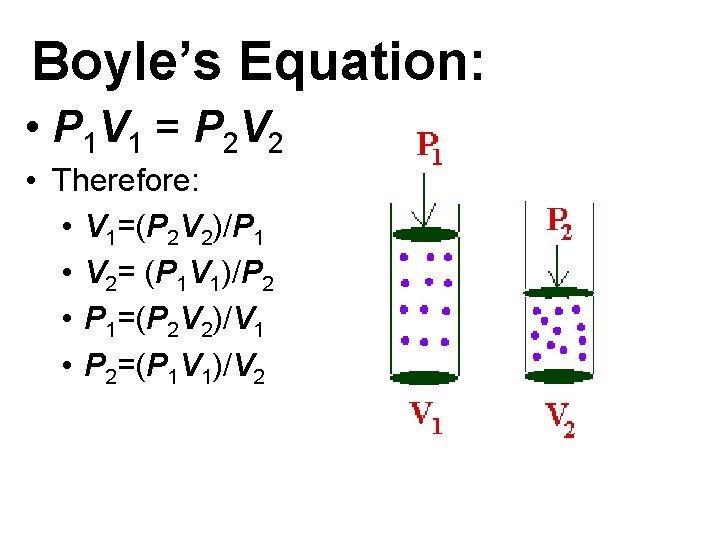
Boyle’s Equation: • P 1 V 1 = P 2 V 2 • Therefore: • V 1=(P 2 V 2)/P 1 • V 2= (P 1 V 1)/P 2 • P 1=(P 2 V 2)/V 1 • P 2=(P 1 V 1)/V 2

Problems 1) You have a 2 L cylinder of gas that is under 100 k. Pa of pressure. If you compress the gas to 1 L, what will the new pressure be? 2) You have a 10. 0 L container of gas under 505 torr of pressure. If you increase the pressure to 1155 torr, what will the new volume be?

The Relationship Between Temperature and Volume

Jaques Charles (1746 -1823) (1746 -1823 • Charles studied the compressibility of gases nearly a century after Boyle • French Physicist • Conducted the first scientific balloon flight in 1783

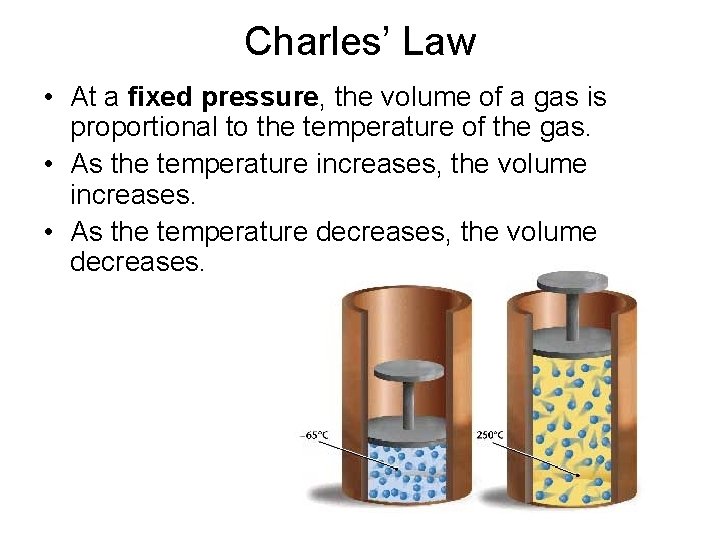
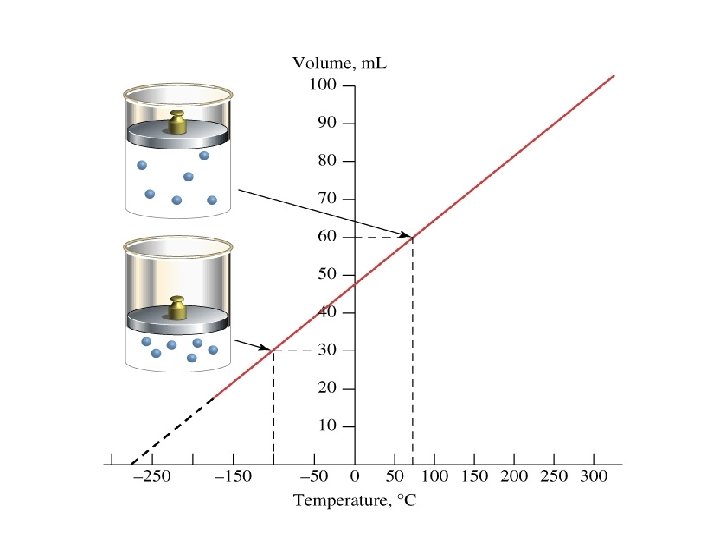
Charles’ Law • At a fixed pressure, the volume of a gas is proportional to the temperature of the gas. • As the temperature increases, the volume increases. • As the temperature decreases, the volume decreases.


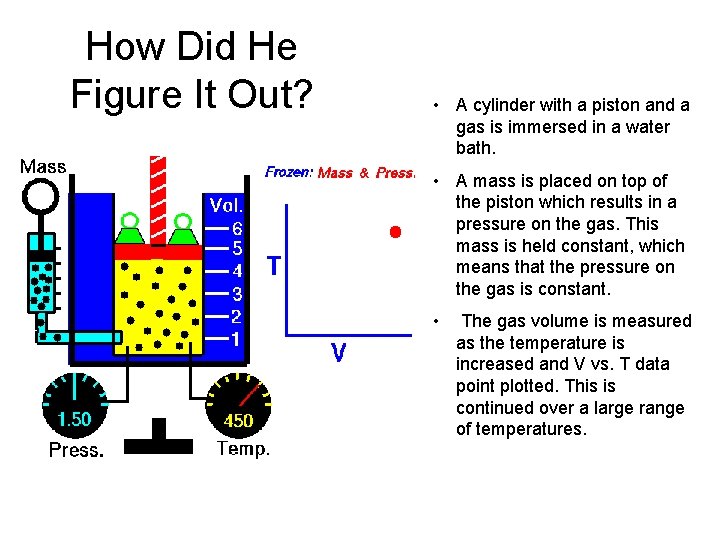
How Did He Figure It Out? • A cylinder with a piston and a gas is immersed in a water bath. • A mass is placed on top of the piston which results in a pressure on the gas. This mass is held constant, which means that the pressure on the gas is constant. • The gas volume is measured as the temperature is increased and V vs. T data point plotted. This is continued over a large range of temperatures.

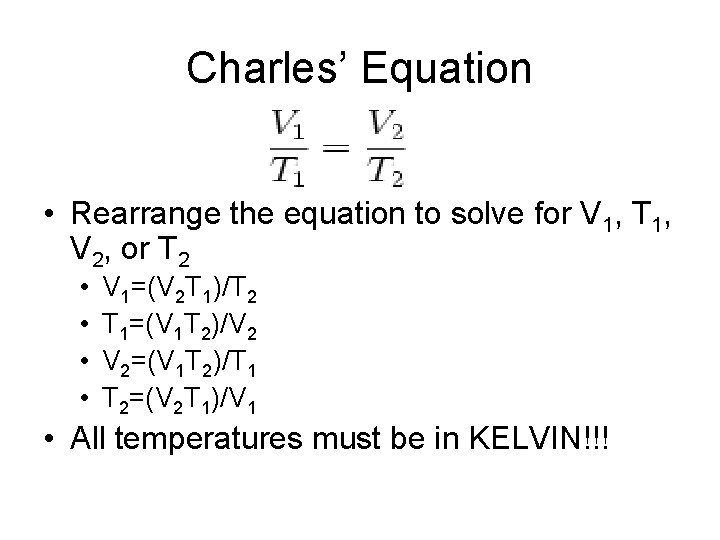
Charles’ Equation • Rearrange the equation to solve for V 1, T 1, V 2, or T 2 • • V 1=(V 2 T 1)/T 2 T 1=(V 1 T 2)/V 2 V 2=(V 1 T 2)/T 1 T 2=(V 2 T 1)/V 1 • All temperatures must be in KELVIN!!!

Problems 1) A cylinder contains 5. 00 L of gas at 225 K. If the temperature is increased to 345 K, what will the new volume be? 2) A tire contains 2. 00 L of air at 300. 0 K, if the volume in the tire decreased to 1. 50 L, what must the new temperature be?

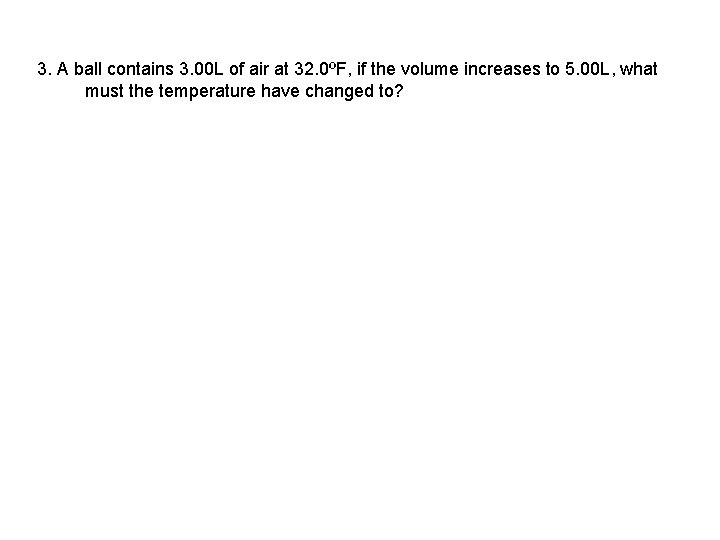
3. A ball contains 3. 00 L of air at 32. 0ºF, if the volume increases to 5. 00 L, what must the temperature have changed to?

Gay-Lussac’s Law The Relationship Between Pressure and Temperature
Joseph Louis Gay-Lussac 1778 - 1850 • French chemist and physicist • Known for his studies on the physical properties of gases. • In 1804 he made balloon ascensions to study magnetic forces and to observe the composition and temperature of the air at different altitudes

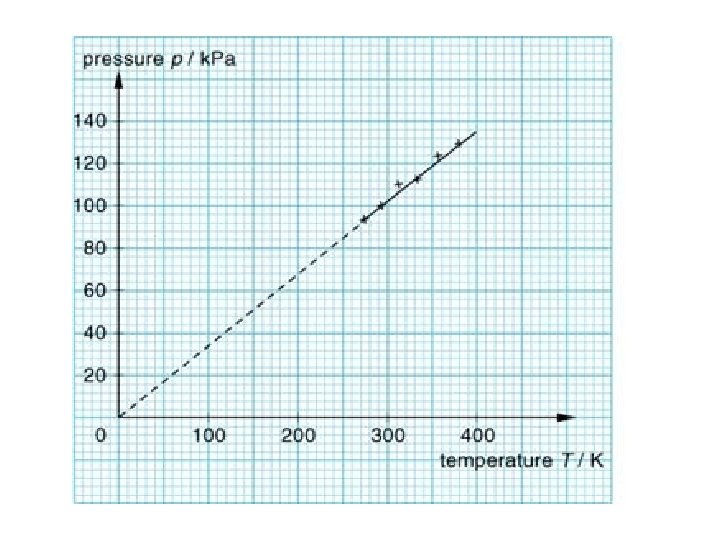
Gay-Lussac’s Law • The pressure of a fixed amount of gas at fixed volume is directly proportional to its temperature in Kelvin. • As the temperature increases, the pressure also increases • As the temperature decreases, the pressure also decreases

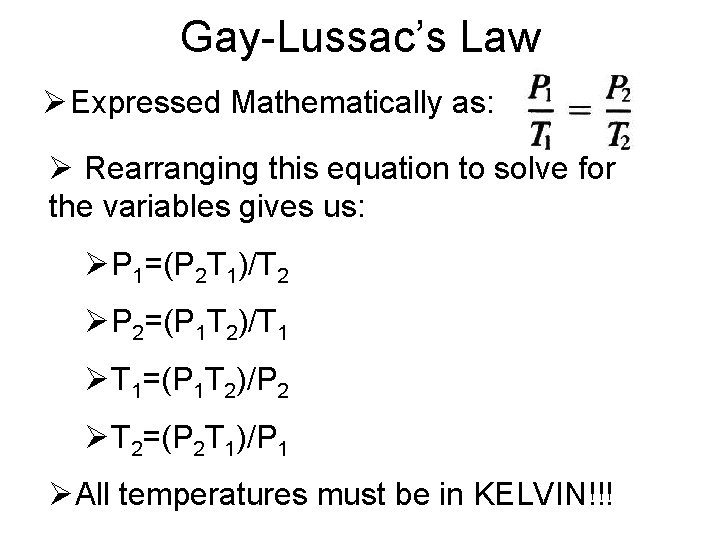
Gay-Lussac’s Law Ø Expressed Mathematically as: Ø Rearranging this equation to solve for the variables gives us: ØP 1=(P 2 T 1)/T 2 ØP 2=(P 1 T 2)/T 1 ØT 1=(P 1 T 2)/P 2 ØT 2=(P 2 T 1)/P 1 ØAll temperatures must be in KELVIN!!!

Problems 1) Jim-Bob has revved his engine enough so that the internal temperature and pressure of his engine are 700. 0 K and 200. 0 k. Pa. If the temperature outside was 295 K before Jim-Bob got into his car, what was the internal pressure in his engine? 2) Today, the barometer reads 750 mm. Hg and thermometer says that it is 65ºF. At 6 am, the barometer read 700 mm. Hg, what was the temperature at that time?

The Combined Gas Law The Relationship Between Pressure, Volume, and Temperature
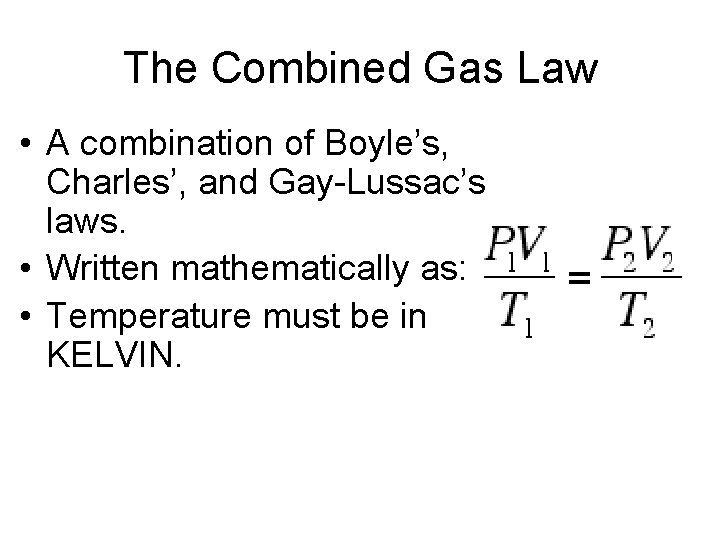
The Combined Gas Law • A combination of Boyle’s, Charles’, and Gay-Lussac’s laws. • Written mathematically as: • Temperature must be in KELVIN.

• Rearranged to solve for each variable: ØP 1=(P 2 V 2 T 1)/(V 1 T 2) ØV 1=(P 2 V 2 T 1)/(P 1 T 2) ØT 1=(P 1 V 1 T 2)/(P 2 V 2) ØP 2=(P 1 V 1 T 2)/(V 2 T 1) ØV 2=(P 1 V 1 T 2)/(P 2 T 1) ØT 2=(P 2 V 2 T 1)/(P 1 V 1)

Problems 1) Rhonda’s helium balloon has a volume of 2. 00 L at 2. 00 atm and 395 K. If the temperature is raised to 500. K and the gas is compressed to 1. 00 L, what is the new pressure? 2) Ryan ate at Taco Shack last night. He now has gas which takes up 1. 29 L of bowel space under 2. 35 atm of pressure at 310 K (body temp). What will be the volume of Jeremy’s gas after he expels it if the atmospheric temperature and pressure are 75ºF and 1. 00 atm respectively?

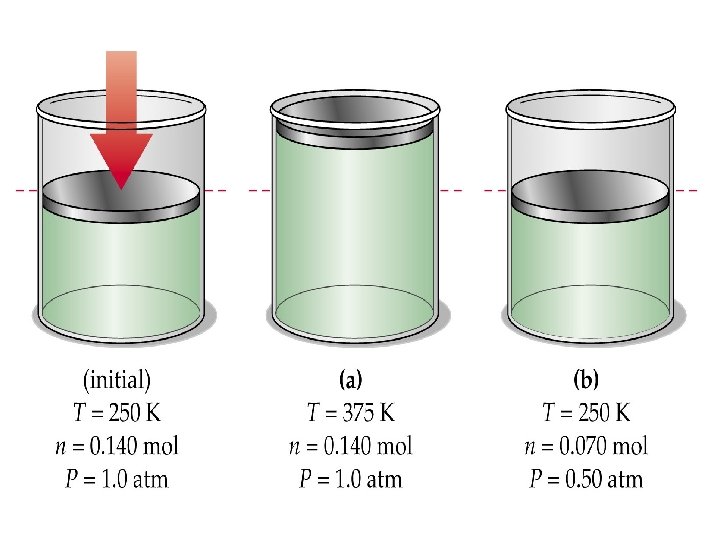
Avogadro’s Law The Relationship Between Pressure, Temperature, Volume and Moles

Amedeo Avogadro (1776 -1856) • Italian physicist and mathematician • Born in a noble ancient family of Piedmont • “Avogadro’s Number” is named after him • 6. 022 x 1023 • The number of things in a mole

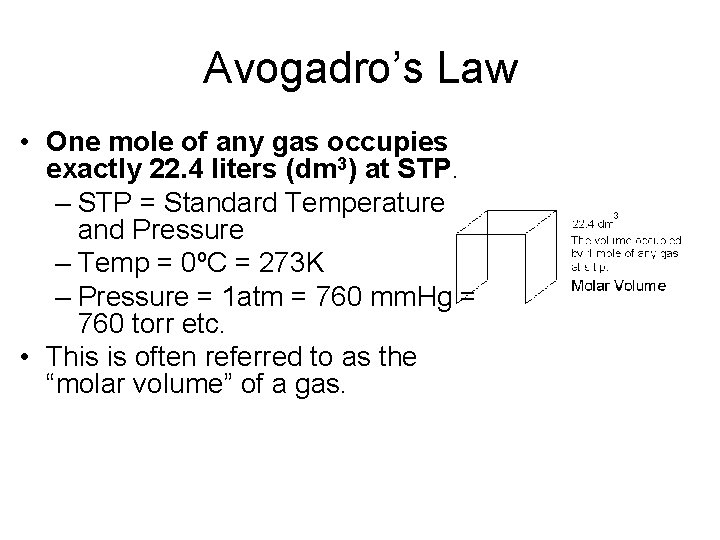
Avogadro’s Law • One mole of any gas occupies exactly 22. 4 liters (dm 3) at STP. – STP = Standard Temperature and Pressure – Temp = 0ºC = 273 K – Pressure = 1 atm = 760 mm. Hg = 760 torr etc. • This is often referred to as the “molar volume” of a gas.

• Equal volumes of gases, at the same temperature and pressure, contain the same number of particles, or molecules. • Thus, the number of molecules in a specific volume of gas is independent of the size or mass of the gas molecules. • Therefore, IT DOESN’T MATTER WHICH GAS YOU ARE TALKING ABOUT.


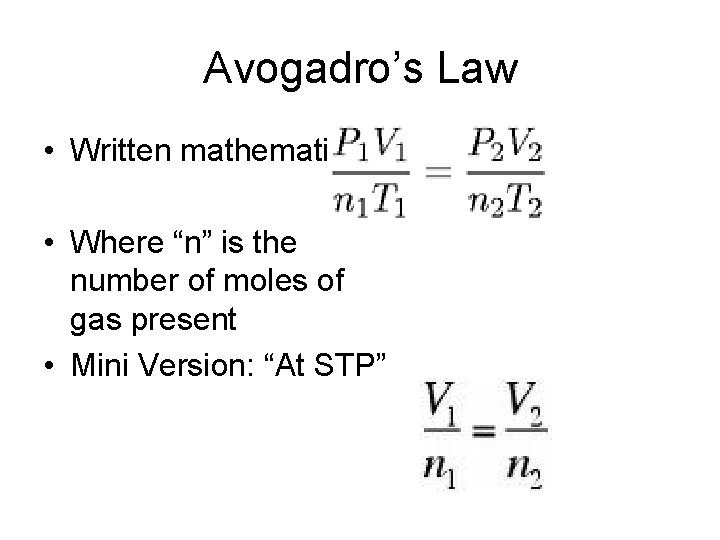
Avogadro’s Law • Written mathematically: • Where “n” is the number of moles of gas present • Mini Version: “At STP”


Problems 1) Luis has 3. 5 mol of H 2 gas at STP. What volume will this gas occupy? 2) Joann has a can of CO 2 gas that contains 0. 180 mol CO 2 at 1 atm and 273 K. If Joann let half of the gas out, what would the new volume be?

More Problems 1) Joe’s balloon contains 1. 0 mole of Helium at STP. If Joe blows another mole of Helium into the balloon, heats it to 350 K, and decreases the volume to 0. 75 L, what will the new pressure be inside the balloon? 2) A 1. 0 L cylinder contains 2. 0 moles of gas at 250 K and 1. 0 atm. If the temperature and pressure are increased to 350 K and 3. 5 atm and the new volume of gas is 0. 50 L, how much gas was added/removed?

The Ideal Gas Law

• The Ideal Gas Law is most often written as: PV=n. RT • Rearranging the equation gives us: ØP = (n. RT)/V ØV = (n. RT)/P Øn = (PV)/(RT) ØT = (PV)/(n. R) Ø All temperatures must be in KELVIN!!!

• R is the Universal Gas Constant ØR = 0. 082 L x atm x K-1 x mol-1 ØR = 62. 36 L x torr x K-1 x mol-1 ØR = 62. 36 L x mm. Hg x K-1 x mol-1 ØR = 8. 315 L x k. Pa x K-1 x mol-1

Problems 1) Rosy has a 5. 0 L tank of H 2 gas. If the pressure inside the tank is 800. 0 torr and the temperature is 300. 0 K, how many moles of hydrogen does her tank contain? 2) Chris’ favorite meal is a bean and cheese burrito from Del Taco. Unfortunately, this means that his intestines were filled with 1. 50 moles of CH 4 gas under 2 atm of pressure. What is the volume of gas that occupies his bowels? *Make an assumption about Temp*

3. Jose wants to cook up some steaks for Super Bowl Sunday. He looks at his BBQ and notices that his Propane (C 3 H 8) tank contains 10. 0 L of the gas under 3. 0 atm at room temperature. How many grams of propane does Jose own?

Dalton’s Law The Law of Partial Pressures

John Dalton (1766 -1844) • English Chemist and Physicist • Did much research on color blindness – He was colorblind • Famous for his atomic theory • Worked with gases

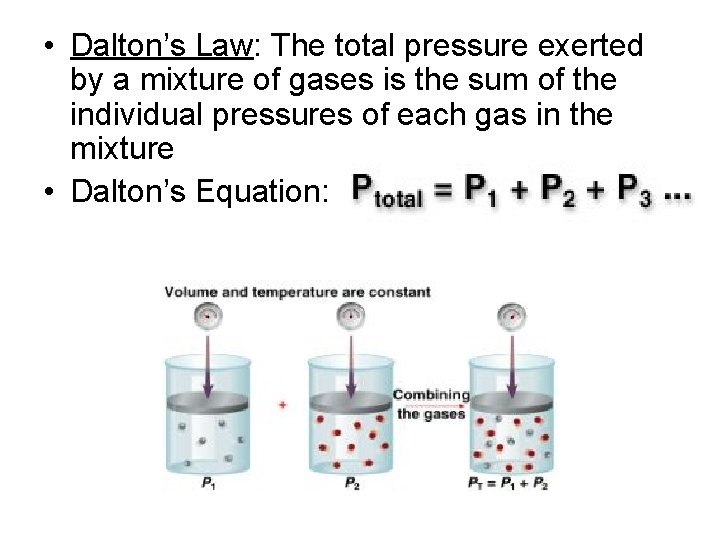
• Dalton’s Law: The total pressure exerted by a mixture of gases is the sum of the individual pressures of each gas in the mixture • Dalton’s Equation:

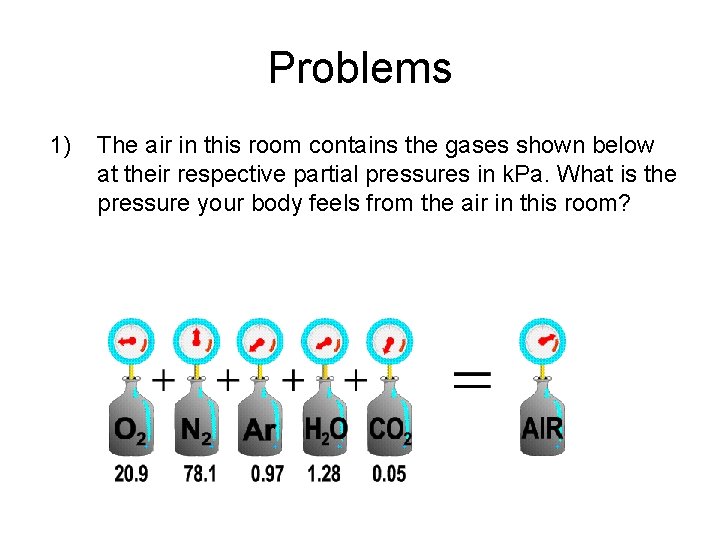
Problems 1) The air in this room contains the gases shown below at their respective partial pressures in k. Pa. What is the pressure your body feels from the air in this room?

2) What is the pressure in problem 1 if you convert it to atm? Torr? mm. Hg? 2) Scuba Divers use gas mixtures of O 2 and He. If the diver to your right breathes in a mixture that has a total pressure of 6. 5 atm and a partial pressure from O 2 of 1. 2 atm, what is the partial pressure of He?

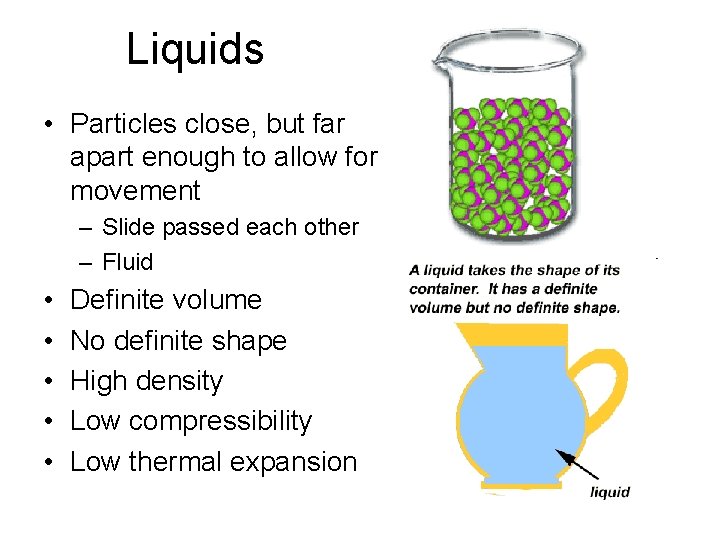
Liquids • Particles close, but far apart enough to allow for movement – Slide passed each other – Fluid • • • Definite volume No definite shape High density Low compressibility Low thermal expansion

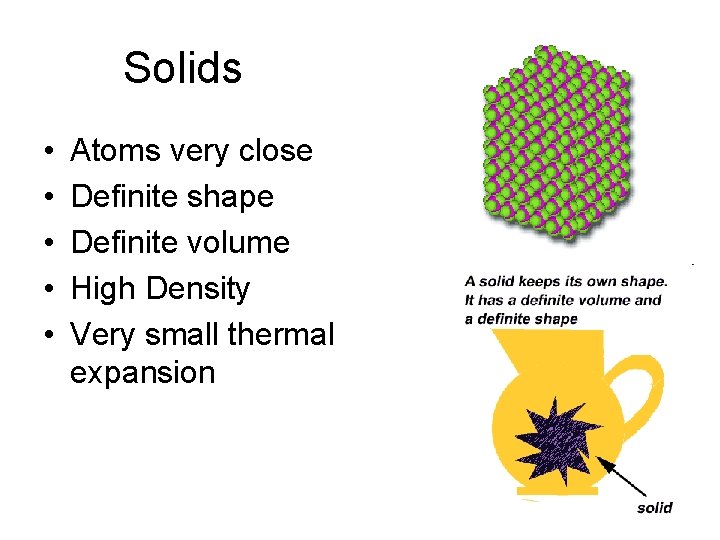
Solids • • • Atoms very close Definite shape Definite volume High Density Very small thermal expansion

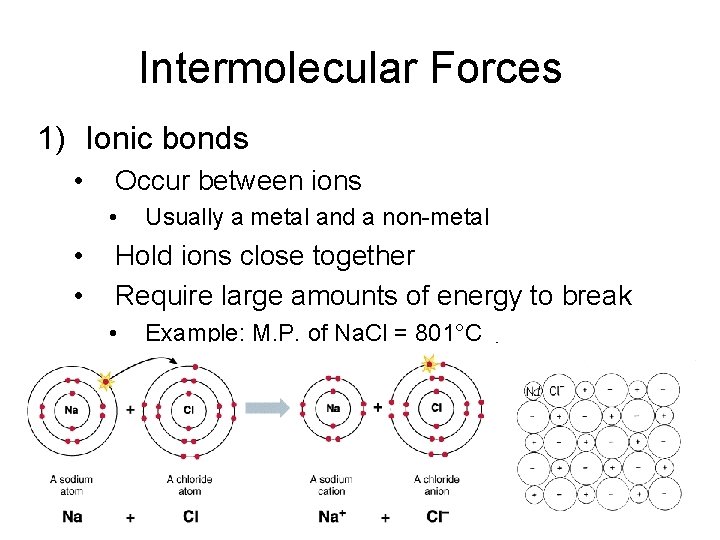
Intermolecular Forces 1) Ionic bonds • Occur between ions • • • Usually a metal and a non-metal Hold ions close together Require large amounts of energy to break • Example: M. P. of Na. Cl = 801°C

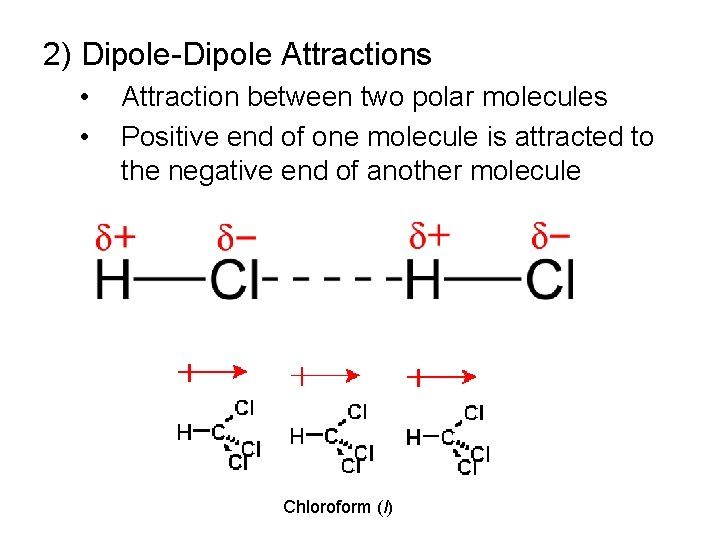
2) Dipole-Dipole Attractions • • Attraction between two polar molecules Positive end of one molecule is attracted to the negative end of another molecule Chloroform (l)


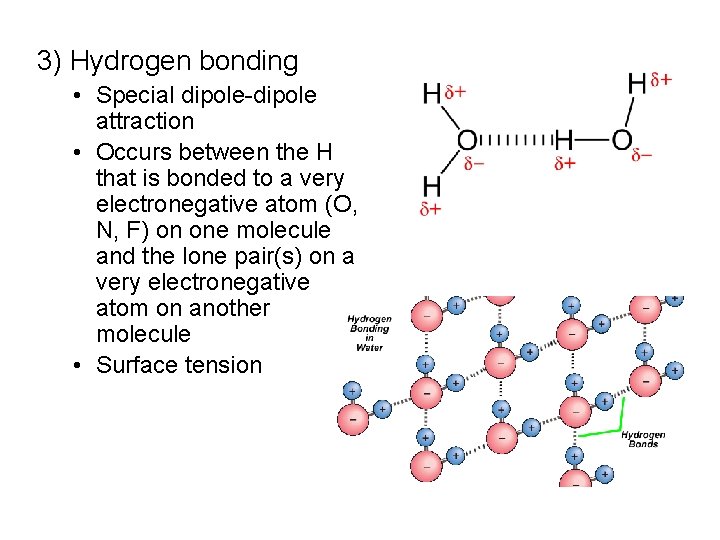
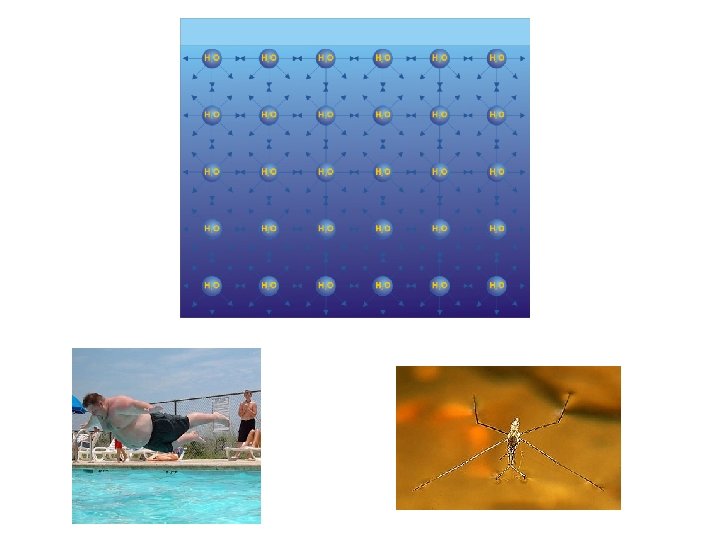
3) Hydrogen bonding • Special dipole-dipole attraction • Occurs between the H that is bonded to a very electronegative atom (O, N, F) on one molecule and the lone pair(s) on a very electronegative atom on another molecule • Surface tension


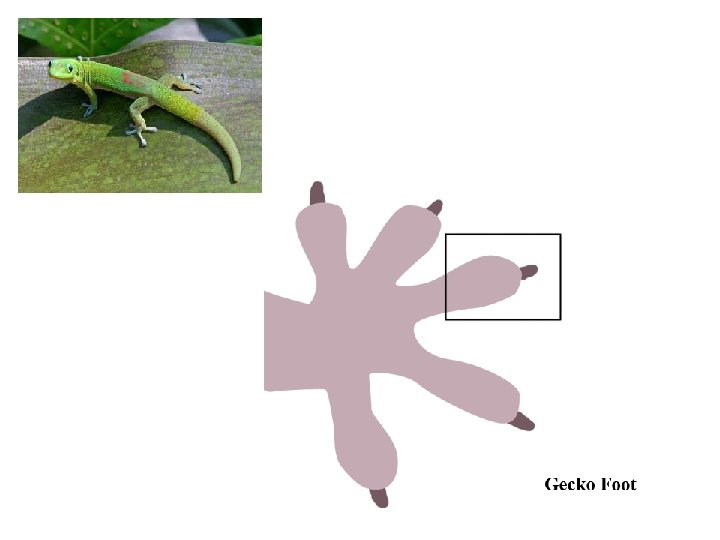
4) Dispersion forces/London forces/Van Der Waals forces • Caused by momentary dipoles in otherwise non-polar molecules • Effect is often seen in very large molecules


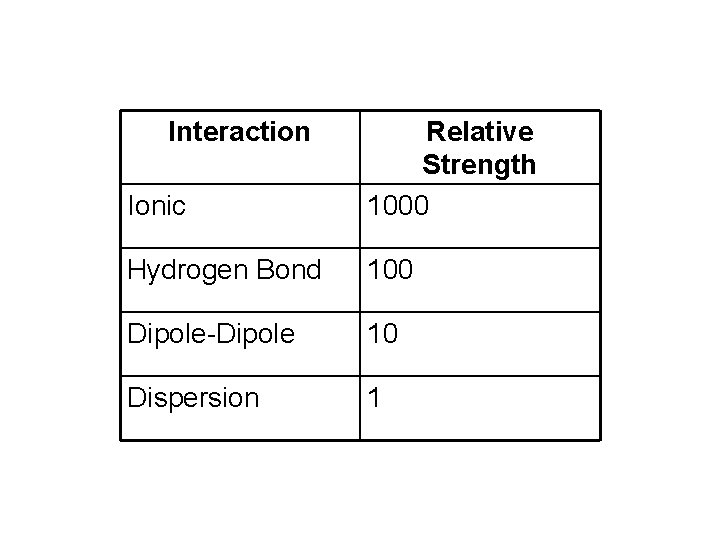
Interaction Ionic Relative Strength 1000 Hydrogen Bond 100 Dipole-Dipole 10 Dispersion 1

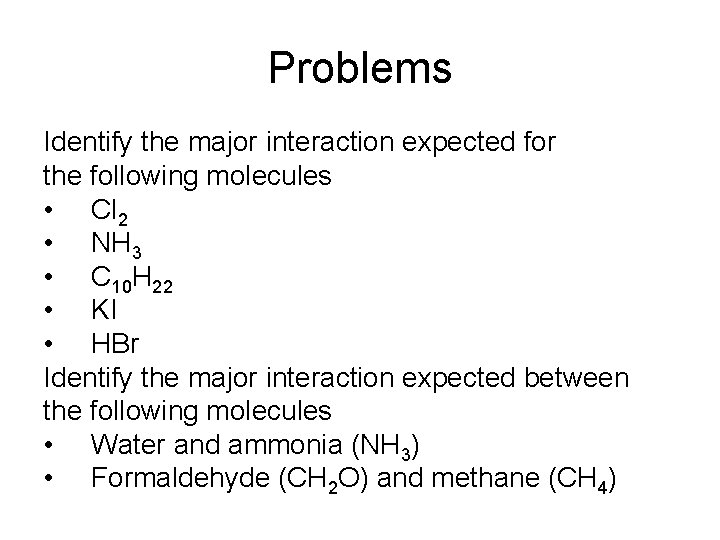
Problems Identify the major interaction expected for the following molecules • Cl 2 • NH 3 • C 10 H 22 • KI • HBr Identify the major interaction expected between the following molecules • Water and ammonia (NH 3) • Formaldehyde (CH 2 O) and methane (CH 4)

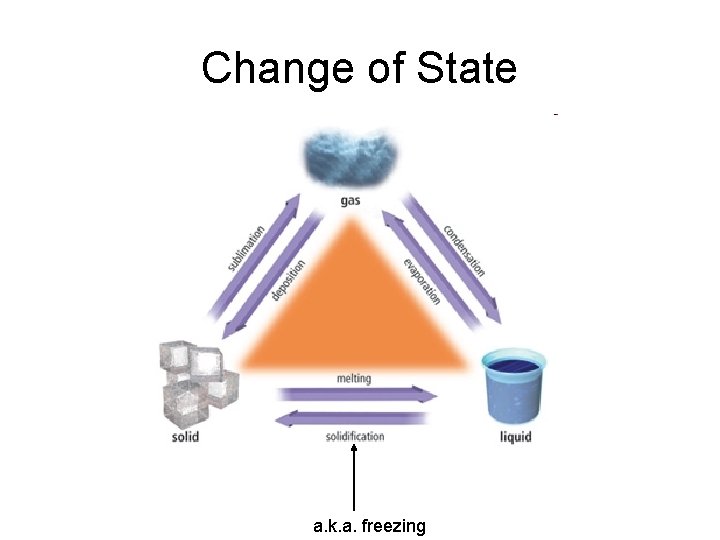
Change of State a. k. a. freezing

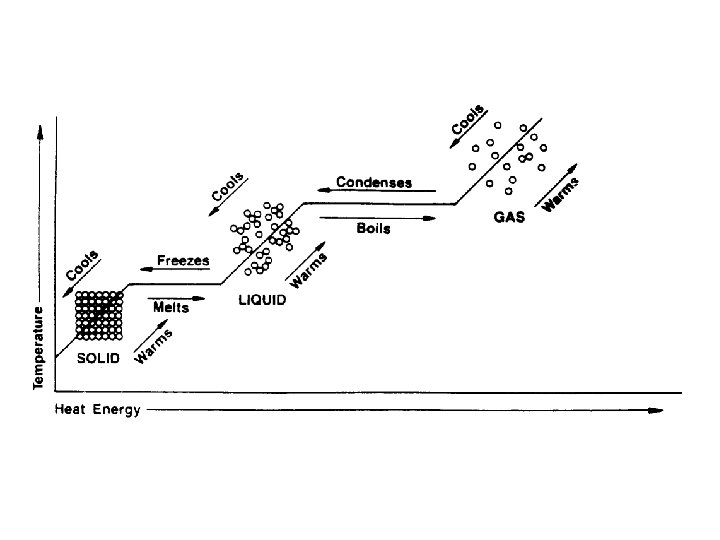
Melting Point (Freezing Point) • The temp at which the solid form of a substance begins to melt and the liquid form of that substance begins to solidify • Most often referred to as M. P. • F. P. understood • Both solid and liquid exists at this temp • Example: Water’s M. P. is 0°C

Boiling Point (Condensation Point) • Temp at which the liquid form of a substance begins to vaporize and the gaseous form of that same substance begins to liquify • Usually referred to as B. P. • C. P. understood • Both liquid and gas occur at this temp


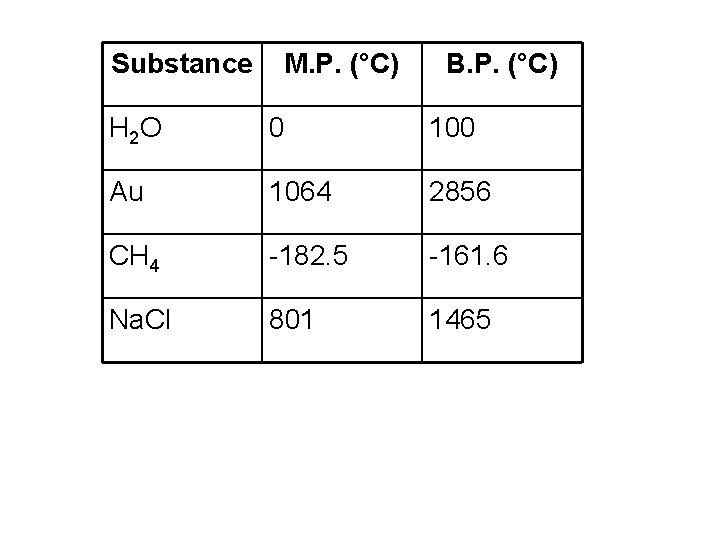
Substance M. P. (°C) B. P. (°C) H 2 O 0 100 Au 1064 2856 CH 4 -182. 5 -161. 6 Na. Cl 801 1465

Chapter 7 review • • Nature of gases Diffusion and Effusion Pressure Boyles, Charles, Gay-Lussacs, Combined, Avogadros, Ideal gas laws. • STP- standard temperature and pressure, 273 K, 1 atm, 1 mole gas = 22. 4 L • Daltons law • Intermolecular Forces – ionic bonds, dipol-dipole, hydrogen-bonding, london dispersion – Know relative strengths!! • Melting/Freezing point and Boiling/condensation point
 Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Kinetic molecular theory of solid
Kinetic molecular theory of solid Expansion of solids liquids and gases examples
Expansion of solids liquids and gases examples Buoyancyability
Buoyancyability Venn diagram solid and liquid
Venn diagram solid and liquid The properties of solids liquids and gases
The properties of solids liquids and gases Why is a gas easier to compress than a liquid or a solid?
Why is a gas easier to compress than a liquid or a solid? Example of solid liquid and gas
Example of solid liquid and gas The science duo physical and chemical changes
The science duo physical and chemical changes Red liquid element
Red liquid element How does sound travel through solids liquids and gases
How does sound travel through solids liquids and gases Properties of solid liquid and gas
Properties of solid liquid and gas Motion of particles in solids, liquids and gases
Motion of particles in solids, liquids and gases Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids Solid to gas
Solid to gas Adhesive force
Adhesive force Filter medium resistance formula
Filter medium resistance formula Liquids and solids menu
Liquids and solids menu Kesler science.com
Kesler science.com Molecular theory of gases and liquids
Molecular theory of gases and liquids State boyle’s law.
State boyle’s law. Chapter 19 liquids exercises answers
Chapter 19 liquids exercises answers Nature and nature's law lay hid in night
Nature and nature's law lay hid in night Determinace lidské psychiky
Determinace lidské psychiky Chapter 11 review gases section 1
Chapter 11 review gases section 1 Chapter 11 review gases section 1
Chapter 11 review gases section 1 Ideal gas law example
Ideal gas law example 14 the behavior of gases
14 the behavior of gases Chapter 13 gases
Chapter 13 gases Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Tư thế worm breton
Tư thế worm breton Hát lên người ơi
Hát lên người ơi Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính thế năng
Công thức tính thế năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thế nào là giọng cùng tên? *
Thế nào là giọng cùng tên? * Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dot
Dot Bảng số nguyên tố lớn hơn 1000
Bảng số nguyên tố lớn hơn 1000 Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Sơ đồ cơ thể người
Sơ đồ cơ thể người