Solids and Liquids Chapter 14 Kinetic Molecular Theory










- Slides: 18

Solids and Liquids Chapter 14

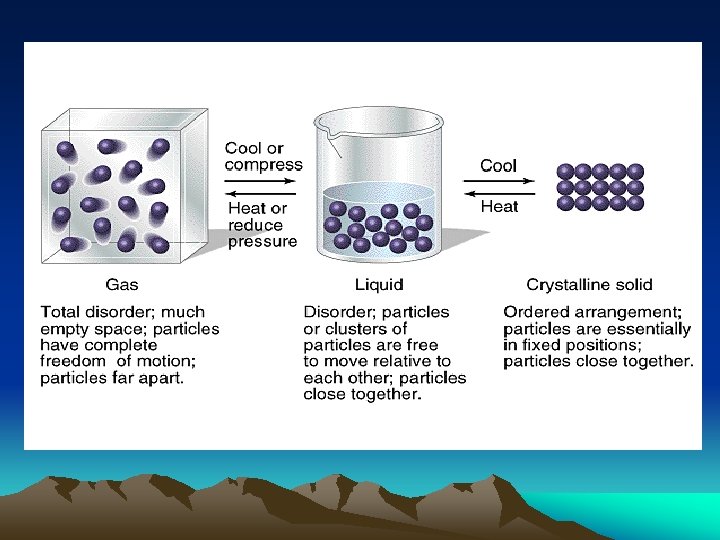
Kinetic Molecular Theory • Solids-particles are highly ordered and packed closely together. They have slight vibrational movement. A solid maintains its shape regardless of the container. • Liquids-particles are more disordered and are spread further than in a solid. They more closely resemble solids than gases.


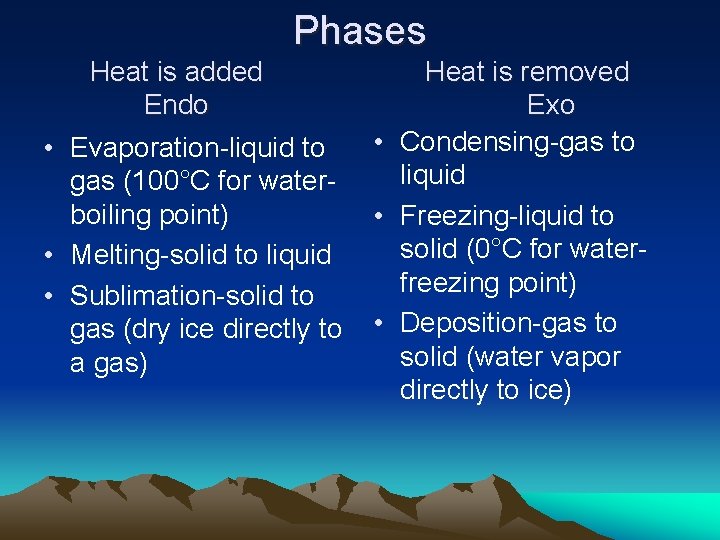
Phases Heat is added Endo • Evaporation-liquid to gas (100°C for waterboiling point) • Melting-solid to liquid • Sublimation-solid to gas (dry ice directly to a gas) Heat is removed Exo • Condensing-gas to liquid • Freezing-liquid to solid (0°C for waterfreezing point) • Deposition-gas to solid (water vapor directly to ice)

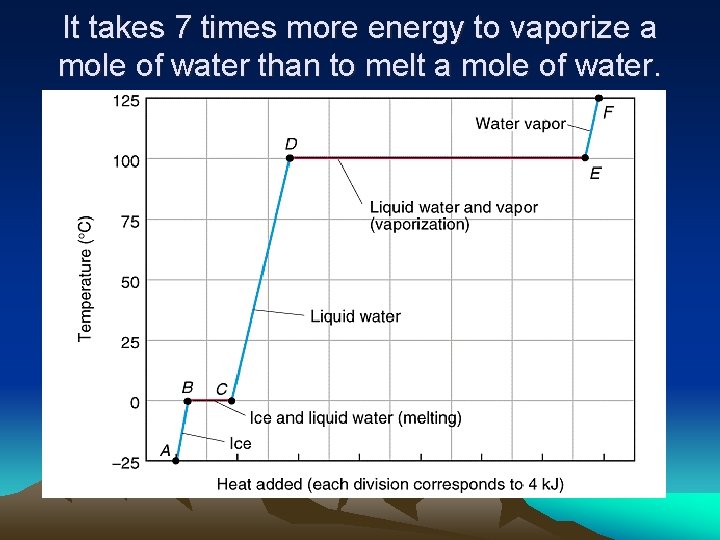
It takes 7 times more energy to vaporize a mole of water than to melt a mole of water.

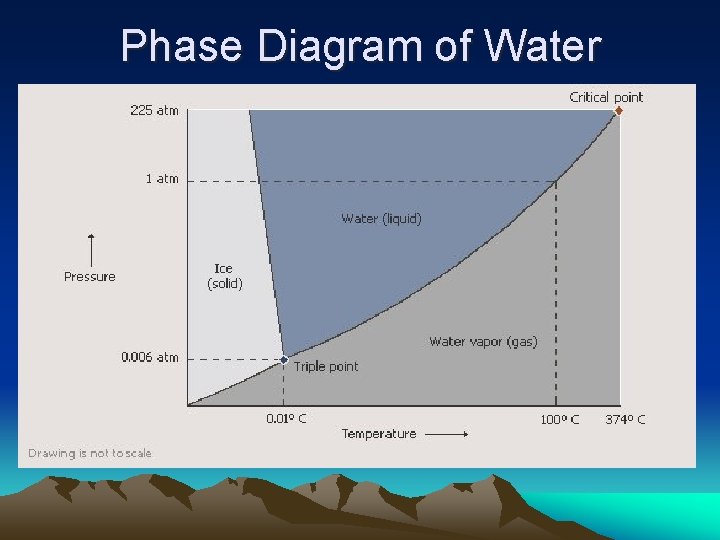
Phase Diagram of Water

Water One gram of ice has a greater volume than one gram of water. liquid water 1. 00 g = 1. 00 g/m. L 1. 00 m. L solid water 1. 00 g = 0. 917 g/m. L 1. 09 m. L

All phase changes are physical changes because a new substance is not created.

What keeps the molecules together? • Intramolecular forces (within the molecule)-holds the atoms of a molecule together Ex: H bonds with O to make H 2 O • Intermolecular forces (between the molecules)-forces between molecules Ex: H 2 O bonds are broken with other H 2 O molecules when making steam.

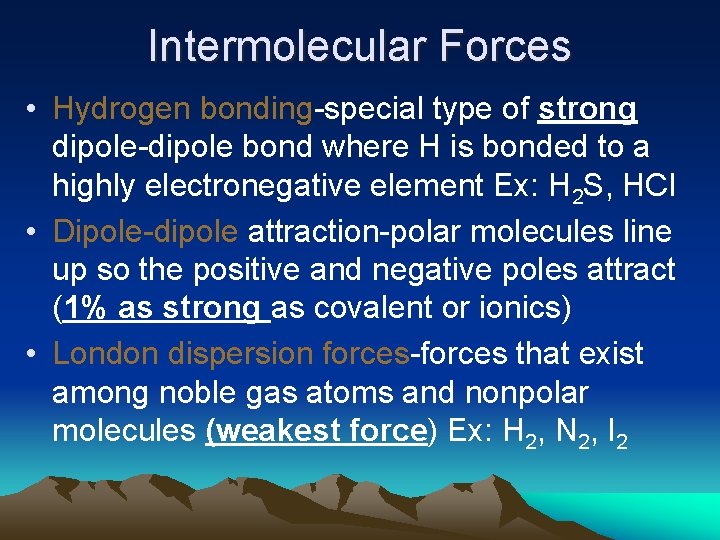
Intermolecular Forces • Hydrogen bonding-special type of strong dipole-dipole bond where H is bonded to a highly electronegative element Ex: H 2 S, HCl • Dipole-dipole attraction-polar molecules line up so the positive and negative poles attract (1% as strong as covalent or ionics) • London dispersion forces-forces that exist among noble gas atoms and nonpolar molecules (weakest force) Ex: H 2, N 2, I 2

Intermolecular Forces: Hydrogen Bonding Forces Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

Types of crystalline solids • Ionic-contains cations and anions, conduct electricity Ex: Salt (Na. Cl). Have high melting points. • Molecular-contains covalent bonds (molecules) Ex: Sugar (C 12 H 22 O 11). Intermolecular forces are weak so they melt at low temperatures. • Atomic-1 element covalently bonded to itself Ex: Diamond (Carbon)

Structure of an Ionic Solid (Na. Cl) Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

Molecular Solids Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

Comparison of a Molecular Compound an Ionic Compound Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

Metallic Solids • Metals have strong, nondirectional bonding. • Electron sea model-metal atoms are located in a sea of valence electrons that are shared • Alloy-a substance that contains a mixture of elements and has metallic properties.

Alloys • Substitutional alloy-metal atoms are replaced by other metal atoms of similar size Ex: sterling silver (93% silver, 7% copper) Analogy: Substitute teacher • Interstitial alloy-some of the interstices (holes) among metal atoms are occupied by smaller atoms Ex: steel (carbon and iron) Analogy: Tutor

Electron Sea Model Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.