Chapter Introduction Lesson 1 Solids Liquids and Gases







































- Slides: 69

Chapter Introduction Lesson 1 Solids, Liquids, and Gases Lesson 2 Changes in State Lesson 3 The Behavior of Gases Chapter Wrap-Up

Describing Matter Two factors determine the state of matter: • the motion of the particles of the matter • the forces between the particles of the matter

Describing Matter (cont. ) • Regardless of how close particles are to each other, they all have random motion; movement in all directions and at different speeds. • Collisions of particles usually change the speed and direction of the particles’ movements.

Describing Matter (cont. ) • In some matter, the particles move slowly. • The particles vibrate in place. • The attractive forces between the particle are strong.

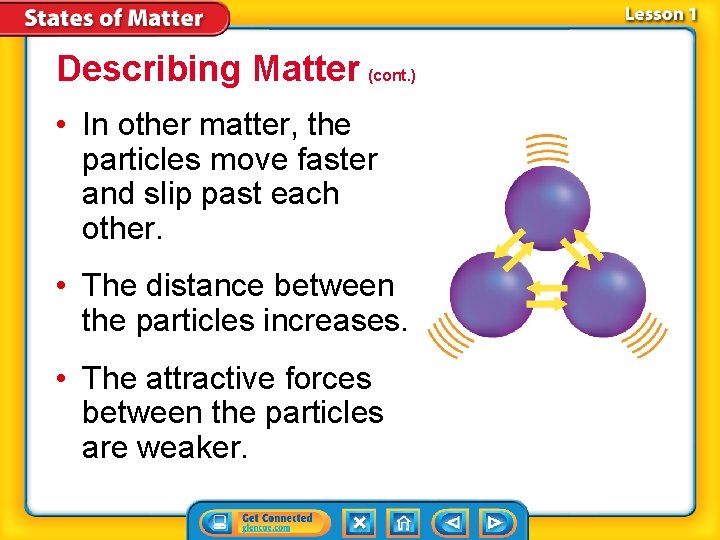
Describing Matter (cont. ) • In other matter, the particles move faster and slip past each other. • The distance between the particles increases. • The attractive forces between the particles are weaker.

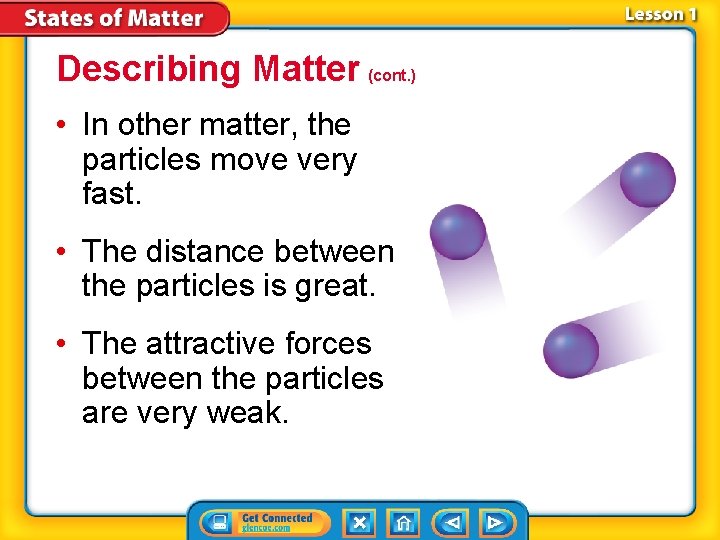
Describing Matter (cont. ) • In other matter, the particles move very fast. • The distance between the particles is great. • The attractive forces between the particles are very weak.

Describing Matter (cont. ) In summary:

Describing Matter (cont. ) • As the motion of the particles slows, the particles move closer. The attractive forces become stronger. • As the motion of the particles increases, the particles move farther apart. The attractive forces become weaker.

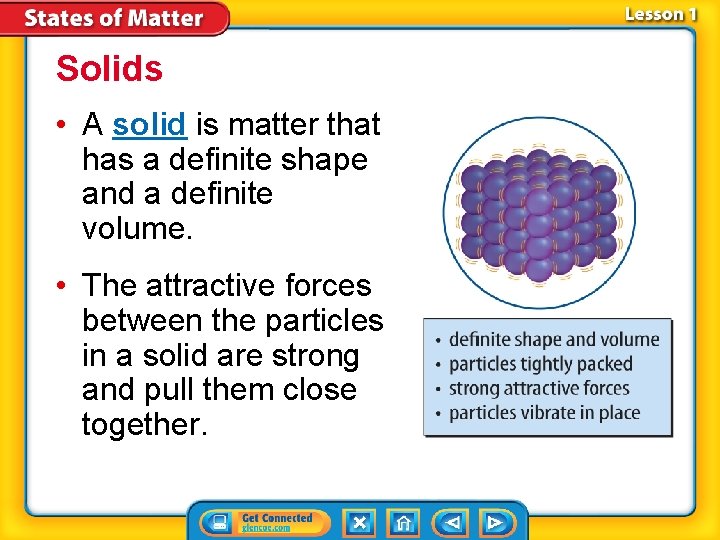
Solids • A solid is matter that has a definite shape and a definite volume. • The attractive forces between the particles in a solid are strong and pull them close together.

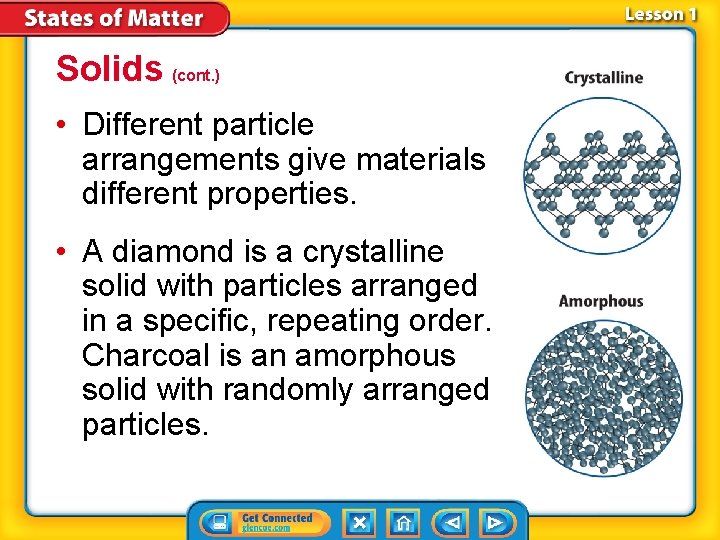
Solids (cont. ) • Different particle arrangements give materials different properties. • A diamond is a crystalline solid with particles arranged in a specific, repeating order. Charcoal is an amorphous solid with randomly arranged particles.

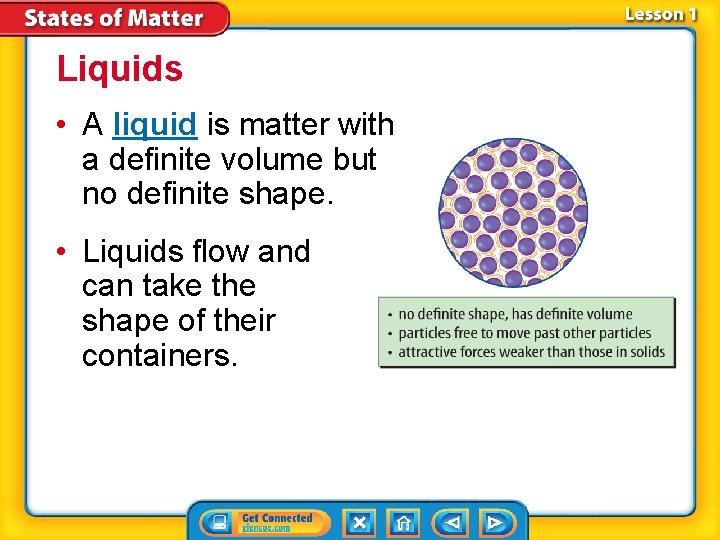
Liquids • A liquid is matter with a definite volume but no definite shape. • Liquids flow and can take the shape of their containers.

Liquids (cont. ) The particle motion in a liquid is faster than the particle motion in a solid.

Liquids (cont. ) Viscosity is a measurement of a liquid’s resistance to flow. Dr. Parvinder Sethi Scott Thomas/Getty Images

Liquids (cont. ) • Molecules at the surface of a liquid have surface tension, the uneven forces acting on the particles on the surface of a liquid. • The surface tension of water enables certain insects to walk on the surface of a lake.

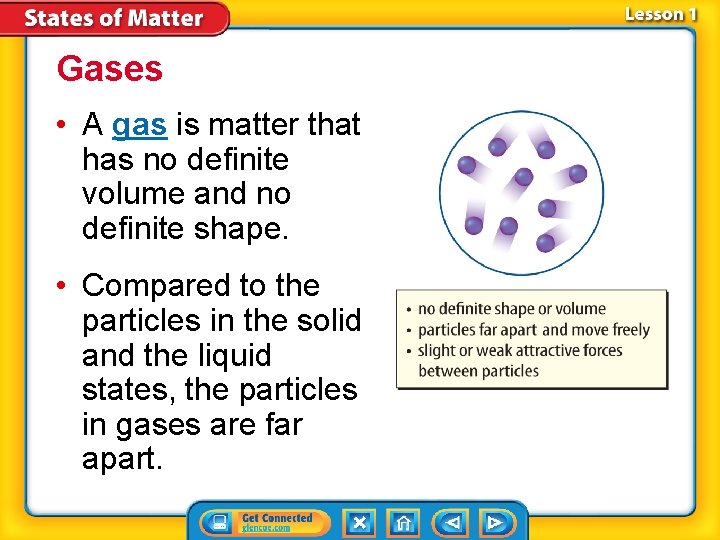
Gases • A gas is matter that has no definite volume and no definite shape. • Compared to the particles in the solid and the liquid states, the particles in gases are far apart.

Gases (cont. ) • In a gas, the forces of attraction between the particles are not strong enough to keep the particles close together. • Because the particles in gas are moving quickly, the distance between particles increases, and the attractive forces between particles decreases. • The gas state of a substance that is normally a solid or a liquid at room temperature is called vapor.

Which describes matter with a definite volume but no definite shape? A. solid B. plasma C. liquid D. gas

Compared to a liquid, which best describes the particles of a gas? A. closer together B. farther apart C. slower moving D. tightly packed

Which term refers to the gas state of a substance that is a solid at room temperature? A. plasma B. surface tension C. vapor D. viscosity

Lesson 2 Changes in State


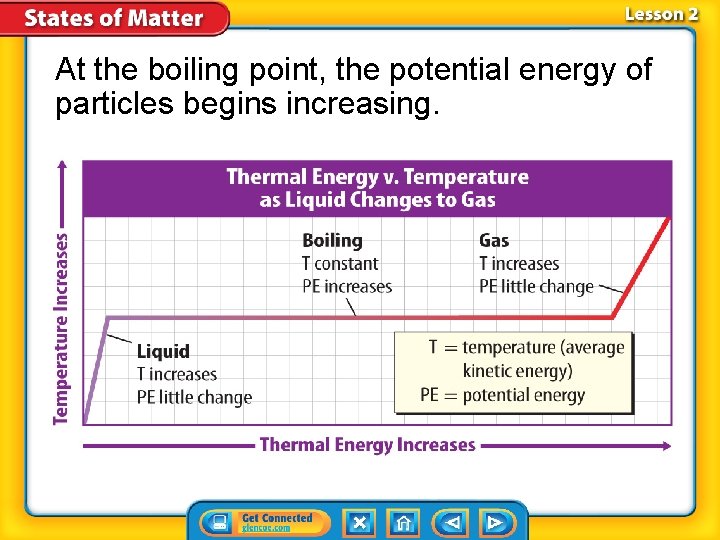
Kinetic and Potential Energy • Particles that make up matter have kinetic energy, the energy an object has due its motion. • The faster particles move, the more kinetic energy they have.

Kinetic and Potential Energy (cont. ) • Temperature: measure of the average kinetic energy of all the particles in an object. • Within a given substance, a temperature increase means that the particles, on average, are moving at greater speeds.

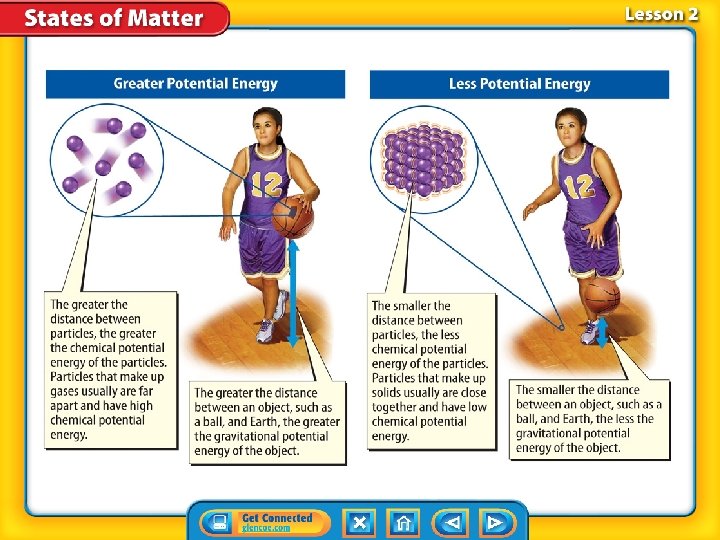
Kinetic and Potential Energy (cont. ) • Potential energy of particles typically increases as the particle get farther apart. • The farther an object is from Earth’s surface, the greater the gravitational potential energy.


Thermal Energy • Thermal energy: total potential and kinetic energy of an object. • You can change an object’s state of matter by adding or removing thermal energy. • If enough thermal energy is added or removed from an object, a change of state can occur.

Solid to Liquid or Liquid to Solid • To change matter from a solid to a liquid, thermal energy must be added. • Once a solid reaches the melting point, additional thermal energy is used by the particles to overcome their attractive forces, the particles move farther apart and potential energy increases.

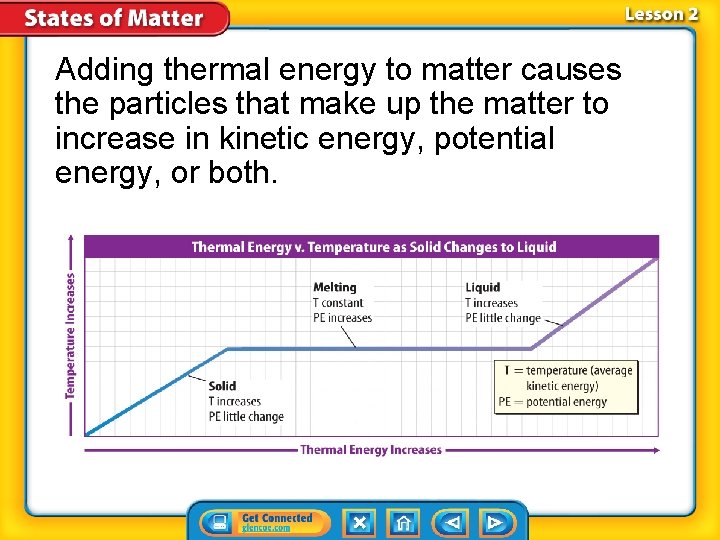
Adding thermal energy to matter causes the particles that make up the matter to increase in kinetic energy, potential energy, or both.

Solid to Liquid or Liquid to Solid (cont. ) • Freezing: process that is the opposite of melting. • Freezing Point: temperature at which matter changes from the liquid state to the solid state

Liquid to Gas or Gas to Liquid • Vaporization: change in state of a liquid into a gas • Boiling: Vaporization that occurs within a liquid • Boiling Point: temperature at which boiling occurs in a liquid

At the boiling point, the potential energy of particles begins increasing.

Liquid to Gas or Gas to Liquid (cont. ) Evaporation: vaporization that occurs only at the surface of a liquid.

Liquid to Gas or Gas to Liquid (cont. ) • When a gas loses enough thermal energy, the gas changes to a liquid, or condenses. • Condensation: change of state from a gas to a liquid

Solid to Gas or Gas to Solid • Sublimation: change of state from a solid to a gas without going through the liquid state. • Deposition: change of state of a gas to a solid without going through the liquid state.

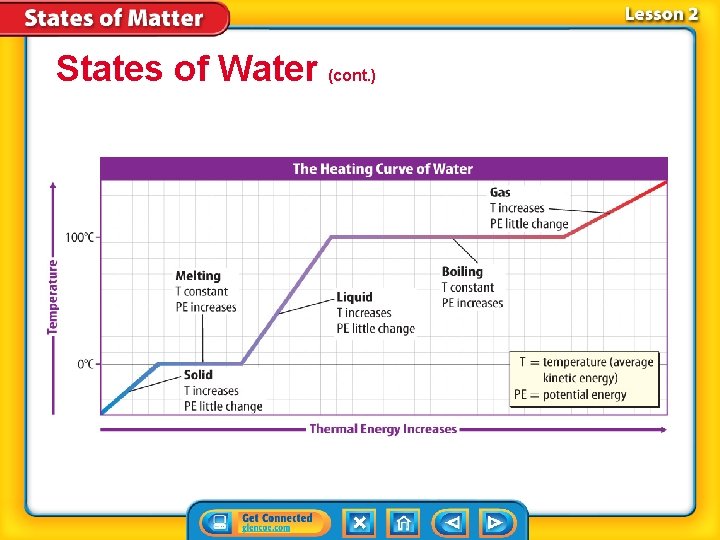
States of Water • Water is the only substance that exists naturally as a solid, a liquid, and a gas on Earth. • At 0°C, water molecules vibrate so rapidly that they begin to move out of their places, the particles overcome their attractive forces, and melting occurs.

States of Water (cont. ) • When water reaches 100°C, the boiling point, liquid water begins to change to water vapor. • Cooling water vapor changes the gas to a liquid, and cooling the water further changes it to ice.

States of Water (cont. )

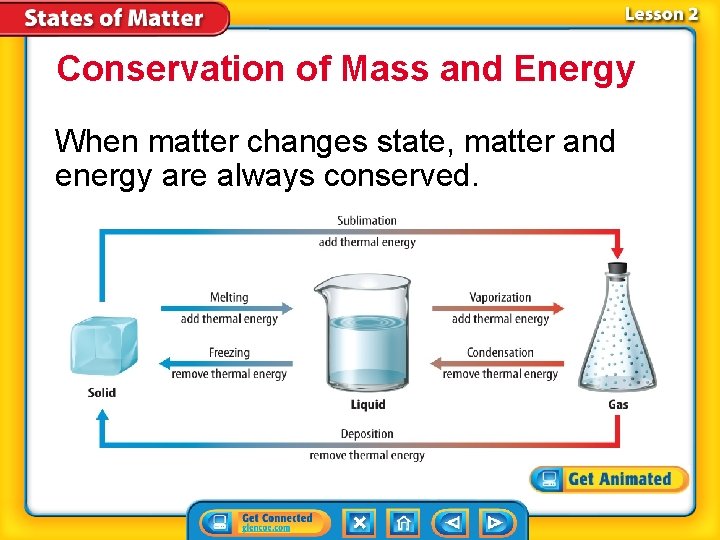
Conservation of Mass and Energy When matter changes state, matter and energy are always conserved.

Which term refers to the average kinetic energy of all the particles in an object? A. thermal energy B. temperature C. sublimation D. evaporation

Which substance exists naturally on Earth as a solid, liquid and gas? A. carbon B. carbon dioxide C. salt D. water

Which term refers to the change of state of a gas to a solid without going through the liquid state? A. sublimation B. evaporation C. deposition D. condensation

Physical and Chemical Changes © The Science Duo

Physical Changes • A substance is altered but it is not transformed into another substance • No change in composition • Only a change in appearance

Examples of Physical Changes • Breaking a glass bottle into tiny pieces • Water evaporating from a puddle • Chopping a pile of wood to use for a fire Breaking Evaporating Chopping

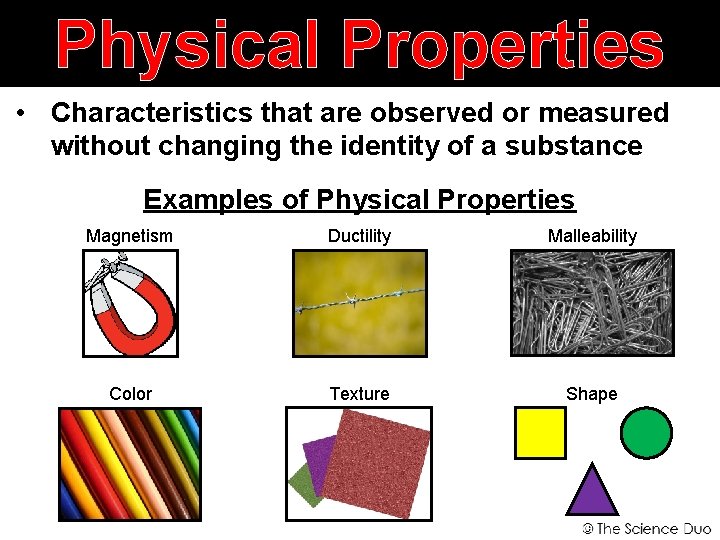
Physical Properties • Characteristics that are observed or measured without changing the identity of a substance Examples of Physical Properties Magnetism Ductility Malleability Color Texture Shape

Chemical Changes • A change that results in the formation of new chemical substances • Change in composition; new substances have different properties • Most chemical changes cannot be reversed

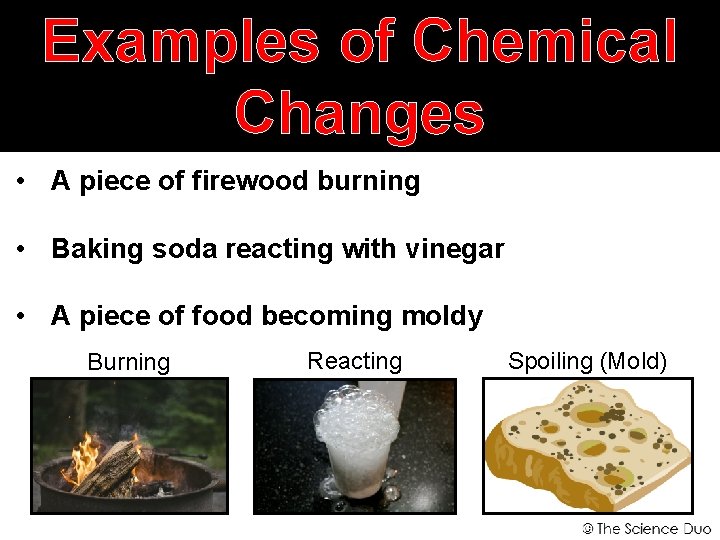
Examples of Chemical Changes • A piece of firewood burning • Baking soda reacting with vinegar • A piece of food becoming moldy Burning Reacting Spoiling (Mold)

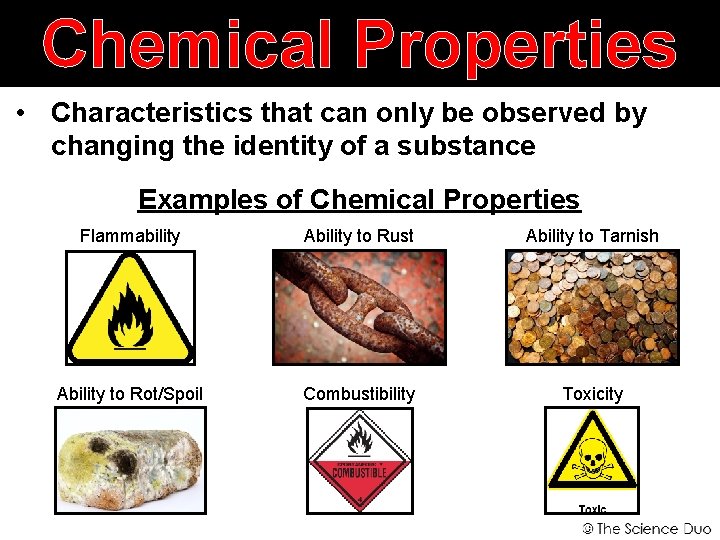
Chemical Properties • Characteristics that can only be observed by changing the identity of a substance Examples of Chemical Properties Flammability Ability to Rust Ability to Tarnish Ability to Rot/Spoil Combustibility Toxicity

Evidence of Chemical Reactions • Unexpected change in energy (heat or light) • Unexpected change in color • Production of gas • Production of precipitate • Change in odor

Lesson 3 The Behavior of Gases

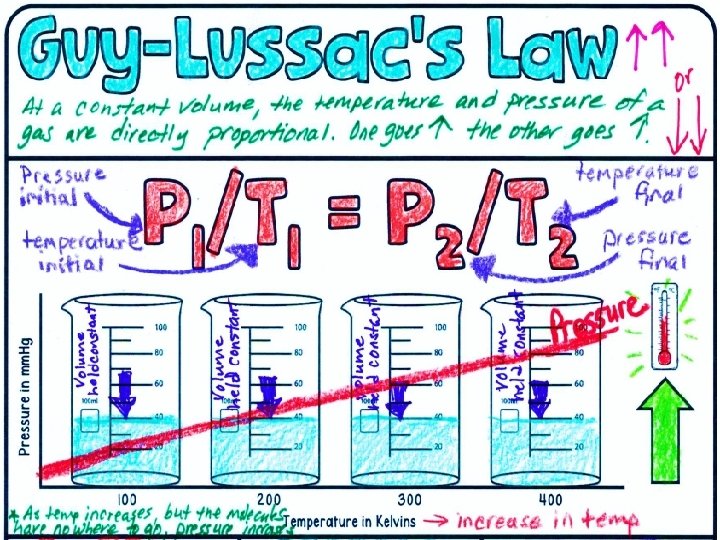
Understanding Gas Behavior The kinetic molecular theory states that the particles in matter collide with other particles, other objects, and the walls of their container; and when particles collide, no energy is lost.

What is pressure? • Pressure: amount of force applied per unit of area. • The empty spaces between particles makes gases compressible.

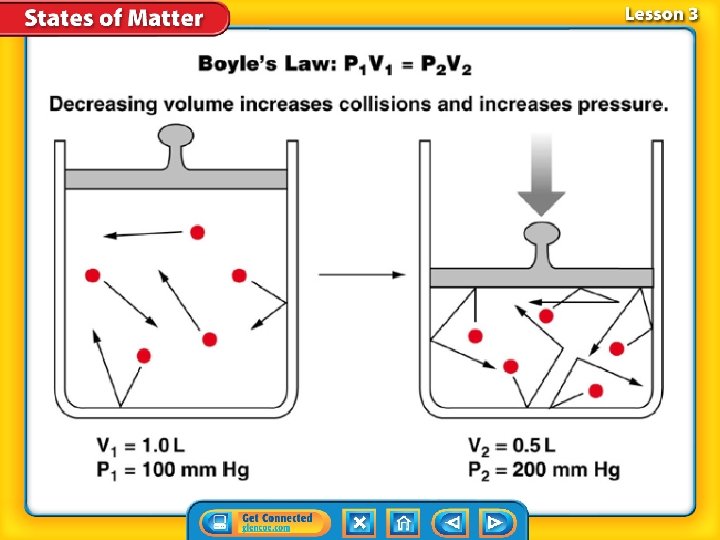
Pressure and Volume When the volume of a container holding gas is greater, the additional space results in fewer collisions and pressure is less.

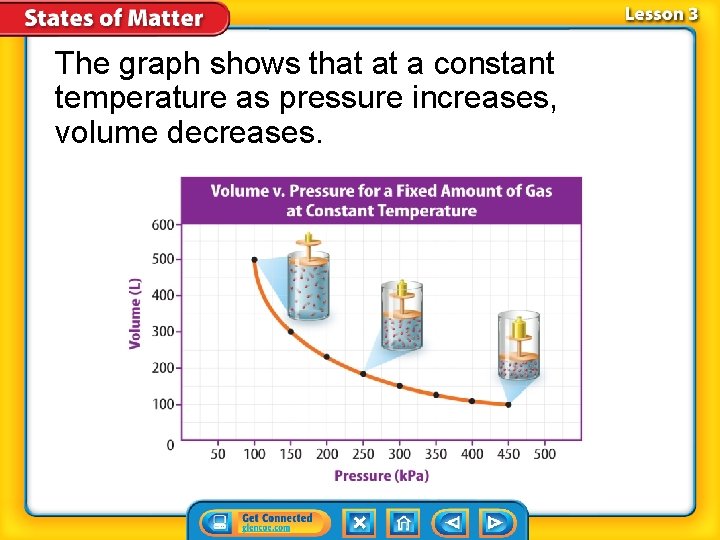
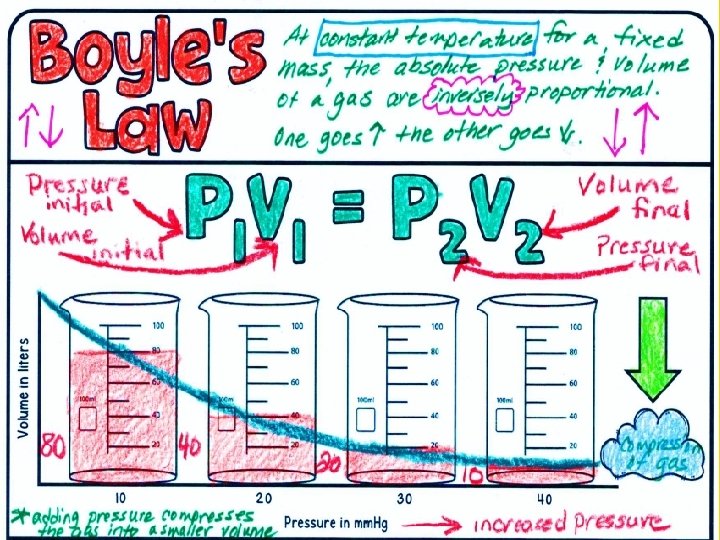
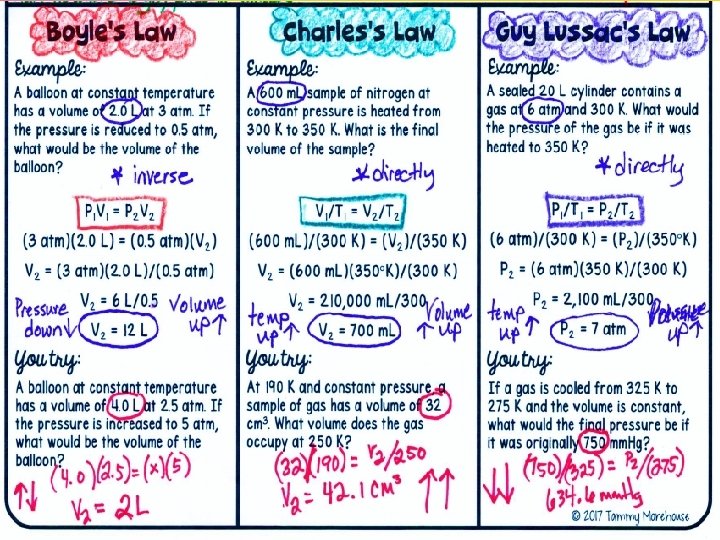
Boyle’s Law Boyle’s law states that pressure of a gas increases if the volume decreases and pressure of a gas decreases if the volume increases, when temperature is constant.

The graph shows that at a constant temperature as pressure increases, volume decreases.


Temperature and Volume • Changing the temperature of a gas affects its behavior. • As the temperature of a gas increases, kinetic energy increases, the particles move farther apart, and volume increases.

Temperature and Volume (cont. )

• https: //www. youtube. com/watch? v=N 5 xft 2 f Iq. QU

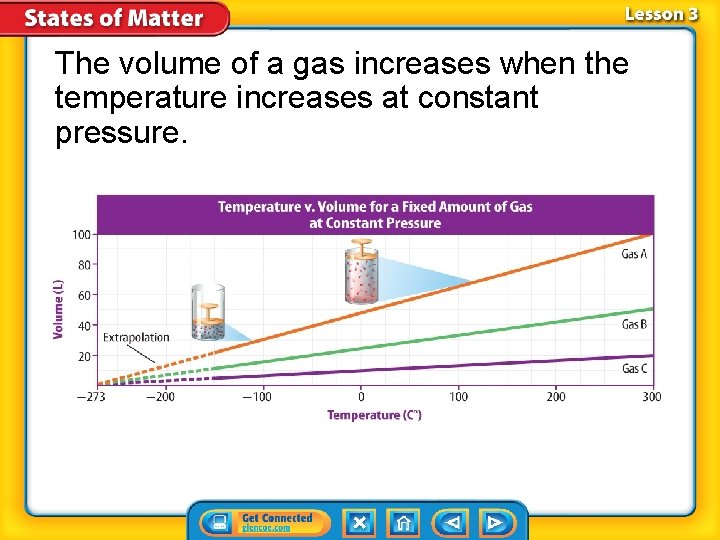
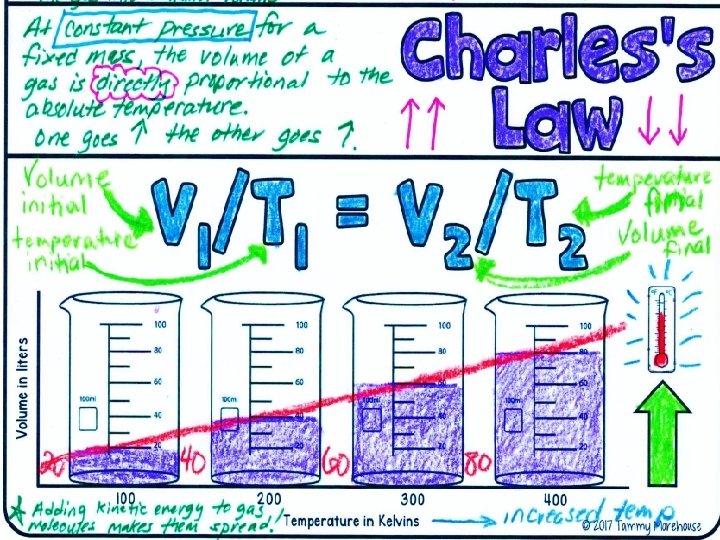
Charles’s Law Charles’s law states that the volume of a gas increases with increasing temperature, if the pressure is constant. --Temperature must be in Kelvin Celsius + 273 = Kelvin

The volume of a gas increases when the temperature increases at constant pressure.





• https: //www. youtube. com/watch? v=Gc. Cm alm. LTi. U

Which states that pressure of a gas increases if the volume decreases? A. Boyle’s law B. Charles’s law C. kinetic molecular theory D. law of thermal energy

As the temperature of a gas increases, what happens to its kinetic energy? A. decreases B. increases C. remains unchanged D. rises and falls

According to the kinetic molecular theory, when particles collide, what happens to energy? A. no energy is lost B. it increases C. it decreases D. all energy is lost