Matter Solids Liquids and Gases START 1 of




































- Slides: 74

Matter Solids, Liquids, and Gases START 1 of 25 20 END © Boardworks Ltd 2005 2004

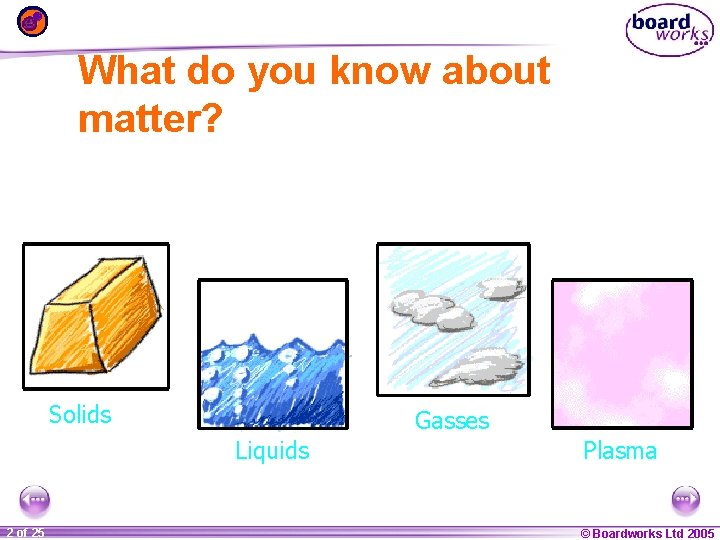
What do you know about matter? Solids Gasses Liquids 1 20 2 of 25 Plasma © Boardworks Ltd 2005 2004

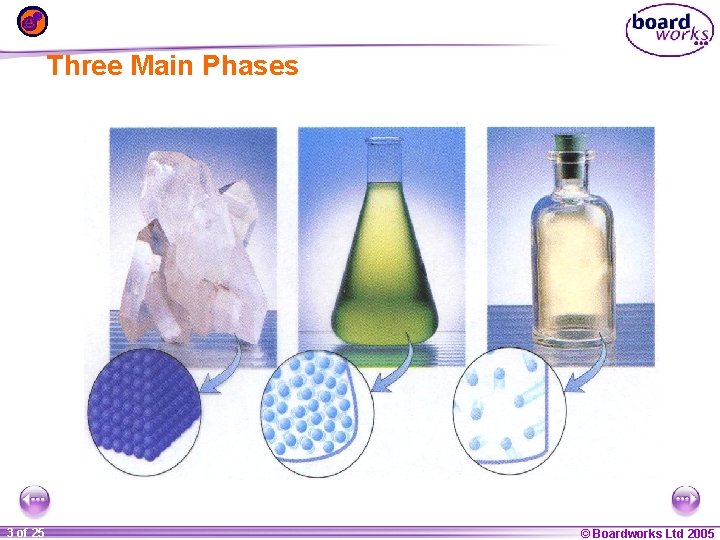
Three Main Phases 1 20 3 of 25 © Boardworks Ltd 2005 2004

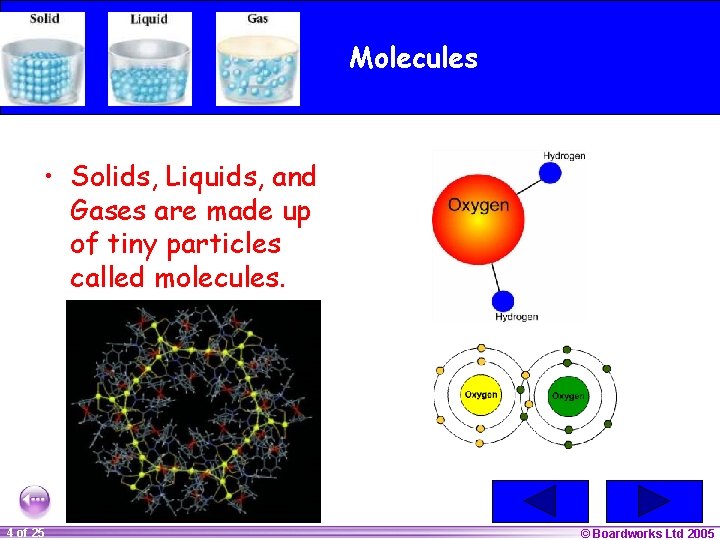
Molecules • Solids, Liquids, and Gases are made up of tiny particles called molecules. 1 20 4 of 25 © Boardworks Ltd 2005 2004

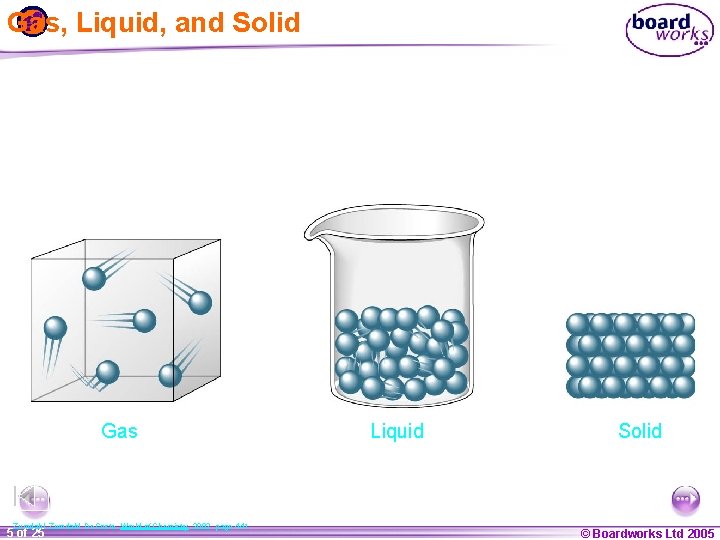
Gas, Liquid, and Solid Gas Zumdahl, De. Coste, World of Chemistry 2002, page 441 1 20 5 of 25 Liquid Solid © Boardworks Ltd 2005 2004

STATES of matter? What would it take for matter to move from one state to another? 1 20 6 of 25 © Boardworks Ltd 2005 2004

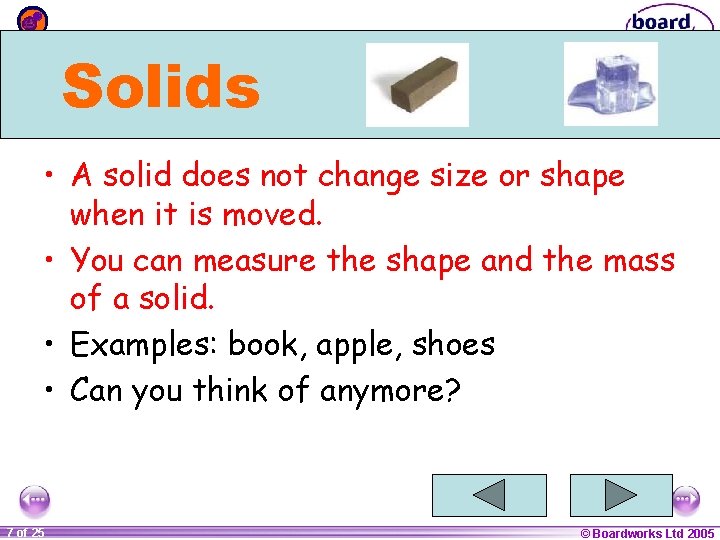
Solids • A solid does not change size or shape when it is moved. • You can measure the shape and the mass of a solid. • Examples: book, apple, shoes • Can you think of anymore? 1 20 7 of 25 © Boardworks Ltd 2005 2004

Solids • The molecules in a solid are very close together and do not move very much. 1 20 8 of 25 © Boardworks Ltd 2005 2004

Solids • Solids hold their own shape. • Solids have weight. • Solids take up space. 1 20 9 of 25 © Boardworks Ltd 2005 2004

Particles in Solids: • Are packed tightly together • Have very little energy 1 10 ofof 20 25 © Boardworks Ltd 2005 2004

• Have a definite shape • Have a definite volume Molecules are held close together and there is very little movement between them. 1 11 ofof 20 25 © Boardworks Ltd 2005 2004

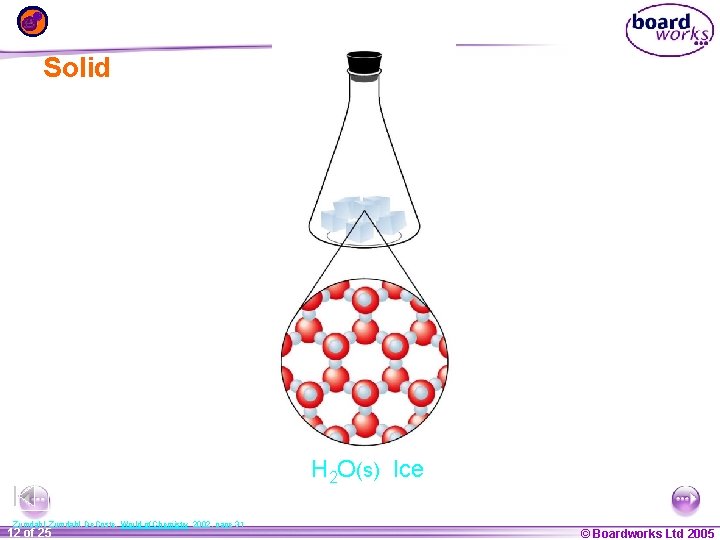
Solid H 2 O(s) Ice Zumdahl, De. Coste, World of Chemistry 2002, page 31 1 12 ofof 20 25 © Boardworks Ltd 2005 2004

Liquids • • 1 13 ofof 20 25 Liquids do not have their own shape. They take the shape of its container. Examples: water, juice, syrup How do liquids look and feel? © Boardworks Ltd 2005 2004

Liquids • Liquids take the shape of their container. • Liquids have weight. • Liquids take up space. 1 14 ofof 20 25 © Boardworks Ltd 2005 2004

Particles in Liquids: • Are loosely packed • Have medium energy levels 1 15 ofof 20 25 © Boardworks Ltd 2005 2004

• Have an indefinite shape • Have a definite volume Atoms and molecules have more space between them than a solid does, but less than a gas (ie. It is more “fluid”. ) 1 16 ofof 20 25 © Boardworks Ltd 2005 2004

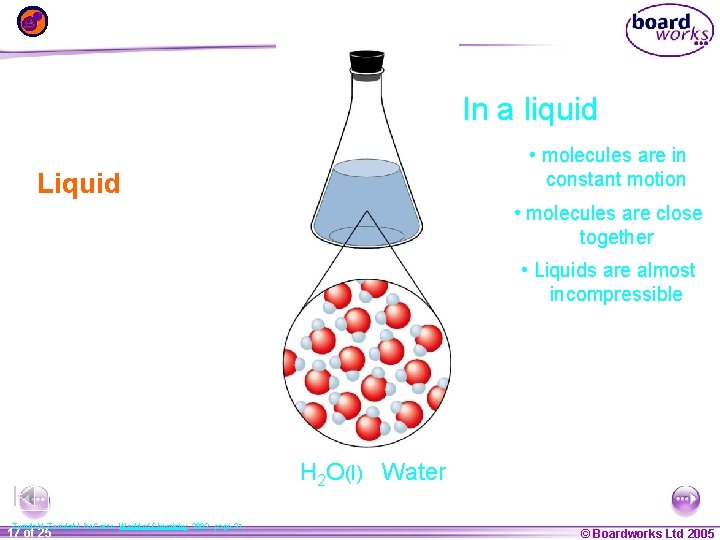
In a liquid • molecules are in constant motion Liquid • molecules are close together • Liquids are almost incompressible H 2 O(l) Water Zumdahl, De. Coste, World of Chemistry 2002, page 31 1 17 ofof 20 25 © Boardworks Ltd 2005 2004

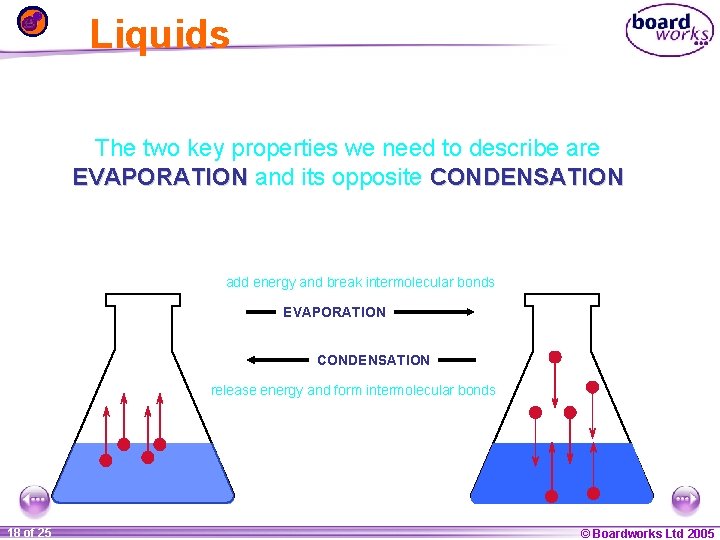
Liquids The two key properties we need to describe are EVAPORATION and its opposite CONDENSATION add energy and break intermolecular bonds EVAPORATION CONDENSATION release energy and form intermolecular bonds 1 18 ofof 20 25 © Boardworks Ltd 2005 2004

Gases • Gas is matter that is all around you and fills many kinds of things. • For example, bubbles, balloons, and balls are filled with gas. • Air is also a gas. • Can you think of another example? 1 19 ofof 20 25 © Boardworks Ltd 2005 2004

Gasses • Gasses spread out to fill the entire space given. • Gasses have weight. • Gasses take up space. 1 20 ofof 20 25 © Boardworks Ltd 2005 2004

Particles in Gasses: • Move freely • Have LOTS of energy 1 21 ofof 20 25 © Boardworks Ltd 2005 2004

• Have an indefinite shape • Have an indefinite volume Molecules are moving in random patterns with different amounts of distance between the particles. 1 22 ofof 20 25 © Boardworks Ltd 2005 2004

Gases • The molecules in a gas have a lot of space between them and move freely. http: //plus. maths. org/issu e 42/features/dartnell/gas_ index. html 1 23 ofof 20 25 © Boardworks Ltd 2005 2004

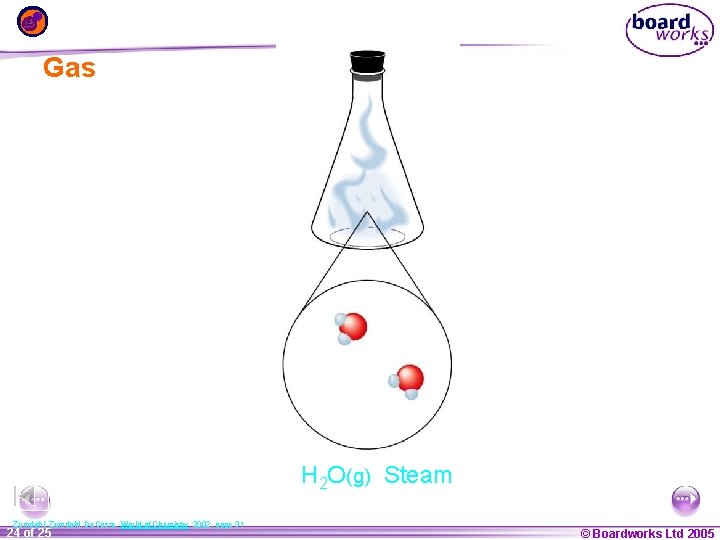
Gas H 2 O(g) Steam Zumdahl, De. Coste, World of Chemistry 2002, page 31 1 24 ofof 20 25 © Boardworks Ltd 2005 2004

But what happens if you raise the temperature to super-high levels… between 1000°C and , 000, 000°C ? Will everything just be a gas? 1 25 ofof 20 25 © Boardworks Ltd 2005 2004

NO! If the gas is made up of particles which carry an electric charge (“ionized particles”), but the entire gas as a whole has no electric charge, and if the density is not too high, then we can get The 4 th state of matter: PLASMA 1 26 ofof 20 25 © Boardworks Ltd 2005 2004

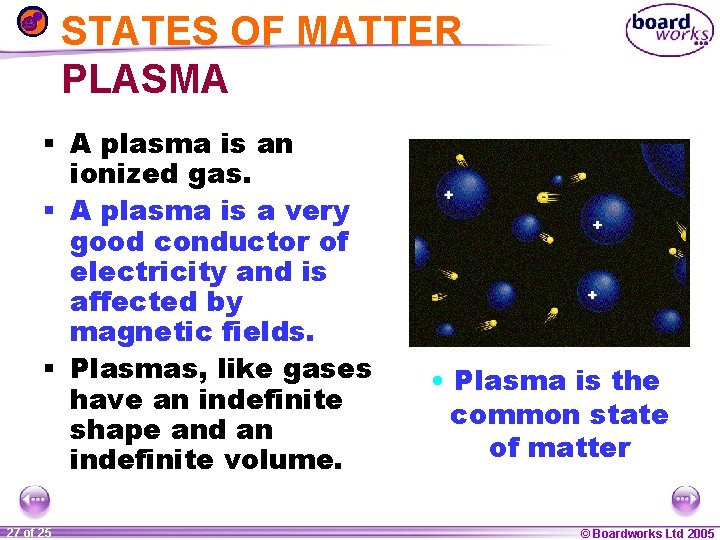
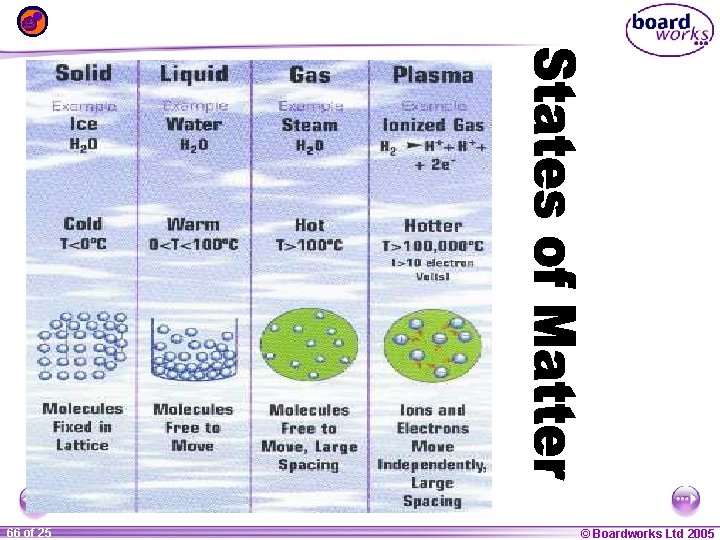
STATES OF MATTER PLASMA § A plasma is an ionized gas. § A plasma is a very good conductor of electricity and is affected by magnetic fields. § Plasmas, like gases have an indefinite shape and an indefinite volume. 1 27 ofof 20 25 • Plasma is the common state of matter © Boardworks Ltd 2005 2004

Some places where plasmas are found… 1. Flames 1 28 ofof 20 25 © Boardworks Ltd 2005 2004

2. Lightning 1 29 ofof 20 25 © Boardworks Ltd 2005 2004

3. Aurora (Northern Lights) 1 30 ofof 20 25 © Boardworks Ltd 2005 2004

4. Neon lights 1 31 ofof 20 25 © Boardworks Ltd 2005 2004

5. Stars make up 99% of the total matter in the Universe. Therefore, 99% of everything that exists in the entire Universe is in the plasma state. 1 32 ofof 20 25 © Boardworks Ltd 2005 2004

The Sun is an example of a star in its plasma state 1 33 ofof 20 25 © Boardworks Ltd 2005 2004

6. Clouds of gas and dust around stars 6 1 34 ofof 20 25 © Boardworks Ltd 2005 2004

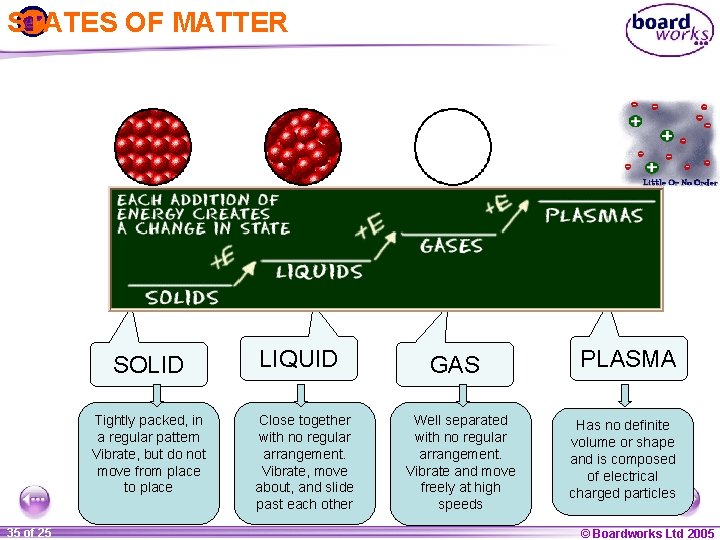
STATES OF MATTER SOLID Tightly packed, in a regular pattern Vibrate, but do not move from place to place 1 35 ofof 20 25 LIQUID Close together with no regular arrangement. Vibrate, move about, and slide past each other GAS Well separated with no regular arrangement. Vibrate and move freely at high speeds PLASMA Has no definite volume or shape and is composed of electrical charged particles © Boardworks Ltd 2005 2004

But now what happens if you lower the temperature way, down to 100 nano degrees above “Absolute Zero” (-273°C) Will everything just be a frozen solid? 1 36 ofof 20 25 © Boardworks Ltd 2005 2004

Not Necessarily! In 1924 (82 years ago), two scientists, Albert Einstein and Satyendra Bose predicted a 5 th state of matter which would occur at very low temperatures. Einstein Bose + 1 37 ofof 20 25 © Boardworks Ltd 2005 2004

Finally, in 1995 (not too long ago), Wolfgang Ketterle and his team of graduate students discovered the 5 th state of matter for the first time. Ketterle and his students The 5 th state of matter: Bose-Einstein Condensate 1 38 ofof 20 25 © Boardworks Ltd 2005 2004

To really understand Bose -Einstein condensate you need to know Quantum Physics 1 39 ofof 20 25 © Boardworks Ltd 2005 2004

In 2002, Ketterle and two other scientists received the highest award in science for discovering Bose-Einstein condensate: The Nobel Prize 1 40 ofof 20 25 © Boardworks Ltd 2005 2004

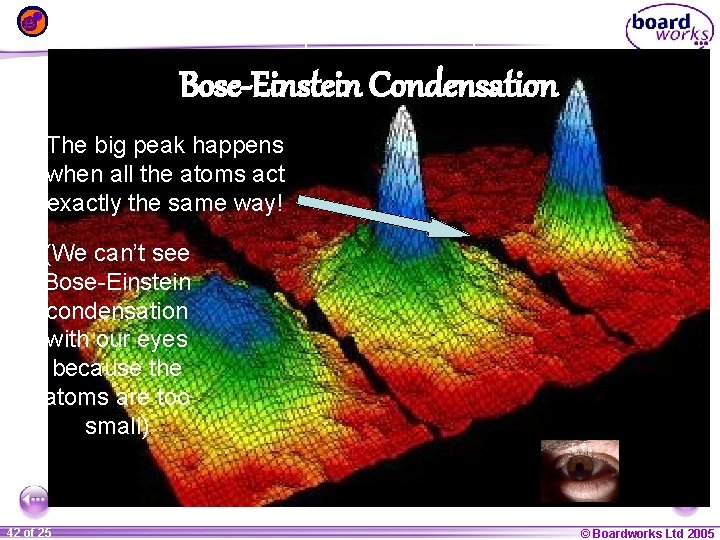
In a Bose-Einstein condensate, atoms can no longer bounce around as individuals. Instead they must all act in exactly the same way, and you can no longer tell them apart! 1 41 ofof 20 25 © Boardworks Ltd 2005 2004

Here is a picture a computer took of Bose-Einstein Condensation The big peak happens when all the atoms act exactly the same way! (We can’t see Bose-Einstein condensation with our eyes because the atoms are too small) 1 42 ofof 20 25 © Boardworks Ltd 2005 2004

Energy and Changes of State • A change of state is the change of a substance from one physical state to another. • A change of state requires a loss or gain of energy by a substance’s particles. 1 43 ofof 20 25 © Boardworks Ltd 2005 2004

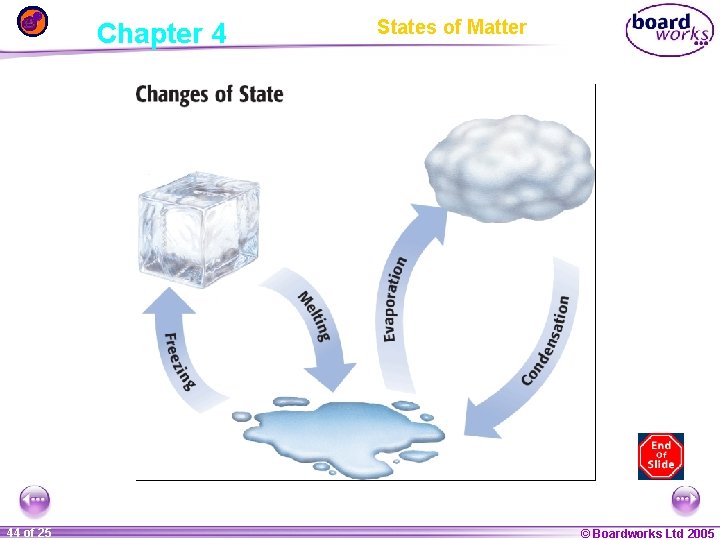
Chapter 4 1 44 ofof 20 25 States of Matter © Boardworks Ltd 2005 2004

Melting: Solid to Liquid • Melting is the change of state in which a solid becomes a liquid by adding heat. • The temperature at which a substance changes from a solid to a liquid is the melting point of the substance. • For a solid to melt, particles must absorb energy. 1 45 ofof 20 25 © Boardworks Ltd 2005 2004

Freezing: Liquid to Solid • Freezing is the change of state from a liquid to a solid. • The temperature at which a liquid changes into a solid is the liquid’s freezing point. • For a liquid to freeze, energy must be removed from the liquid to slow the movement of the particles. 1 46 ofof 20 25 © Boardworks Ltd 2005 2004

Evaporation: Liquid to Gas • Evaporation is the change of state from a liquid to a gas. • Boiling is the change of a liquid to a vapor, or gas. The temperature at which this change happens is the boiling point. • Water boils more easily if the atmospheric pressure is lower. 1 47 ofof 20 25 © Boardworks Ltd 2005 2004

Condensation: Gas to Liquid • Condensation is the change of state from a gas to a liquid. Condensation and evaporation are the reverse of each other. • The condensation point is the temperature at which a gas becomes a liquid. • For condensation to occur, energy must be removed from the gas to slow the movement of the particles. 1 48 ofof 20 25 © Boardworks Ltd 2005 2004

Sublimation: Solid to Gas • Sublimation is the change of state in which a solid changes directly to a gas. • In sublimation, the particles must go from being very tightly packed to being spread far apart. • For sublimation to occur, the solid must gain energy for the particles to overcome their attractions. 1 49 ofof 20 25 © Boardworks Ltd 2005 2004

On earth we live upon an island of "ordinary" matter. The different states of matter generally found on earth are solid, liquid, and gas. We have learned to work, play, and rest using these familiar states of matter. Sir William Crookes, an English physicist, identified a fourth state of matter, now called plasma, in 1879. 1 50 ofof 20 25 © Boardworks Ltd 2005 2004

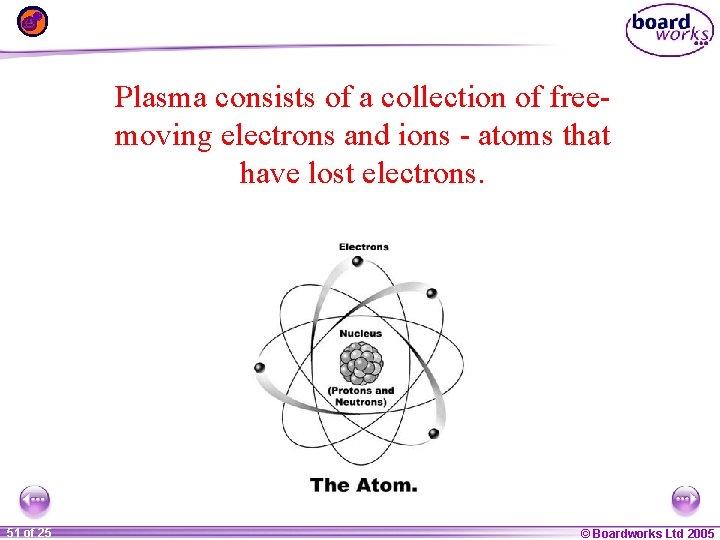
Plasma consists of a collection of freemoving electrons and ions - atoms that have lost electrons. 1 51 ofof 20 25 © Boardworks Ltd 2005 2004

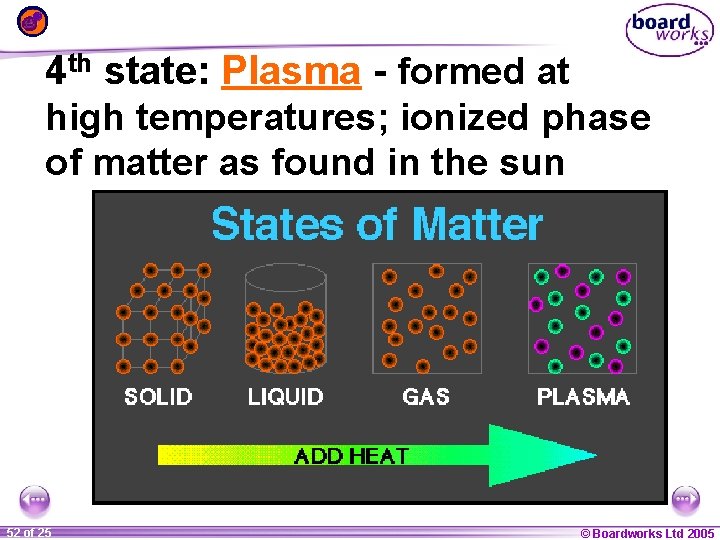
4 th state: Plasma - formed at high temperatures; ionized phase of matter as found in the sun 1 52 ofof 20 25 © Boardworks Ltd 2005 2004

1 53 ofof 20 25 © Boardworks Ltd 2005 2004

1 54 ofof 20 25 © Boardworks Ltd 2005 2004

1 55 ofof 20 25 © Boardworks Ltd 2005 2004

1 56 ofof 20 25 © Boardworks Ltd 2005 2004

1 57 ofof 20 25 © Boardworks Ltd 2005 2004

Can you think of an object that can act as a solid, liquid, and a gas? 1 58 ofof 20 25 © Boardworks Ltd 2005 2004

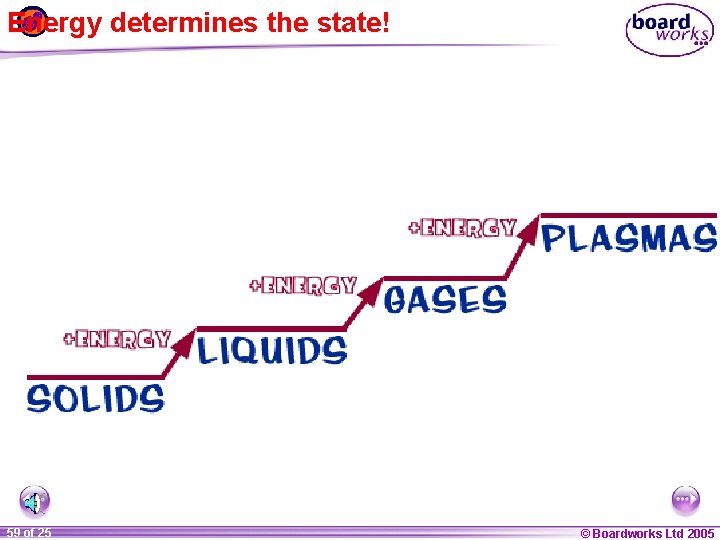
Energy determines the state! 1 59 ofof 20 25 © Boardworks Ltd 2005 2004

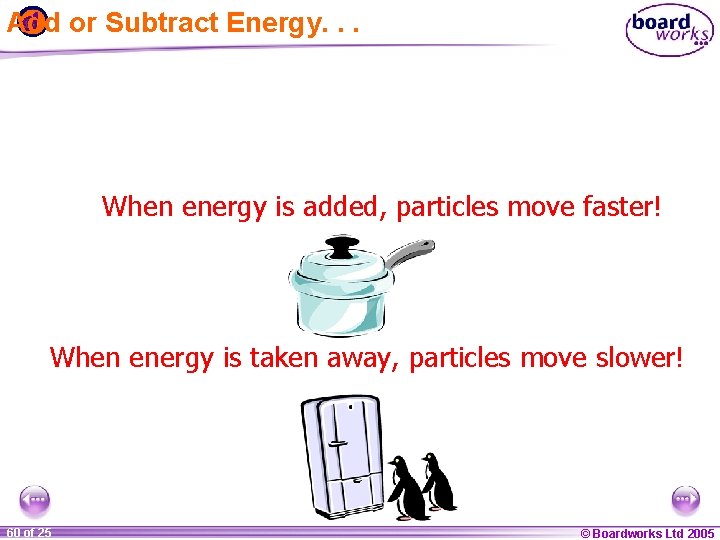
Add or Subtract Energy. . . When energy is added, particles move faster! When energy is taken away, particles move slower! 1 60 ofof 20 25 © Boardworks Ltd 2005 2004

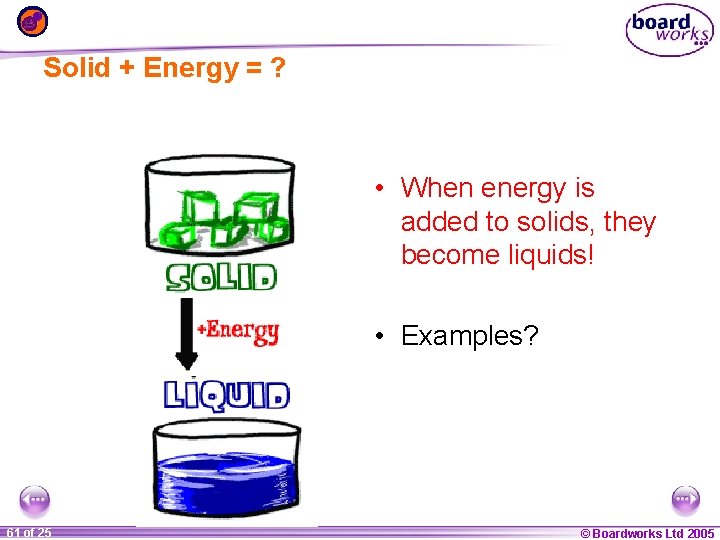
Solid + Energy = ? • When energy is added to solids, they become liquids! • Examples? 1 61 ofof 20 25 © Boardworks Ltd 2005 2004

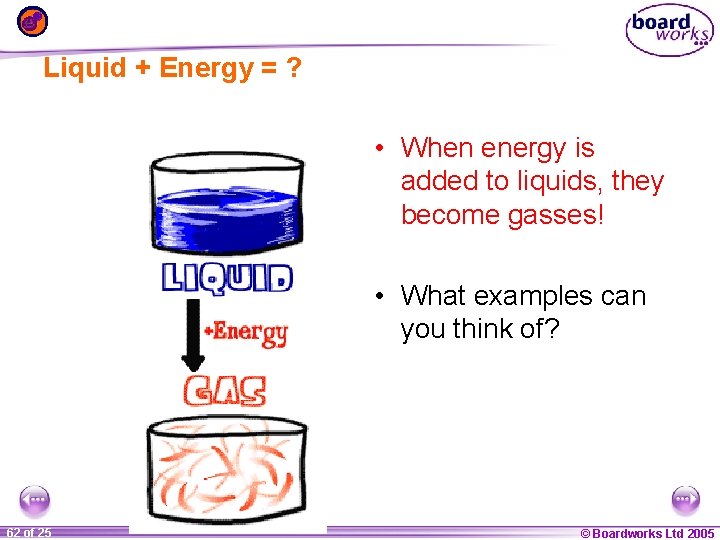
Liquid + Energy = ? • When energy is added to liquids, they become gasses! • What examples can you think of? 1 62 ofof 20 25 © Boardworks Ltd 2005 2004

Solid, Liquid, Gas (a) Particles in solid 1 63 ofof 20 25 (b) Particles in liquid (c) Particles in gas © Boardworks Ltd 2005 2004

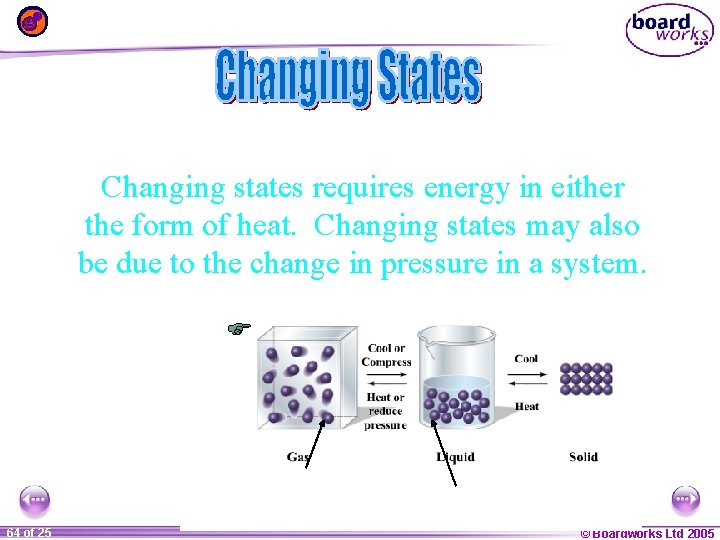
Changing states requires energy in either the form of heat. Changing states may also be due to the change in pressure in a system. 1 64 ofof 20 25 © Boardworks Ltd 2005 2004

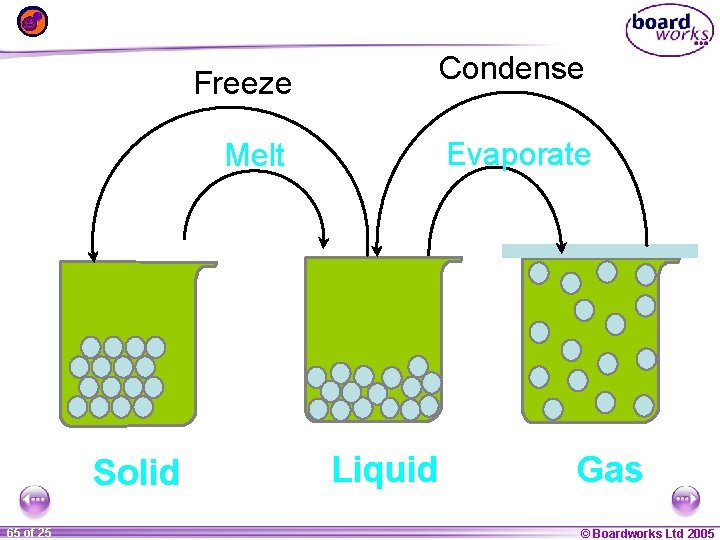
Solid 1 65 ofof 20 25 Freeze Condense Melt Evaporate Liquid Gas © Boardworks Ltd 2005 2004

1 66 ofof 20 25 © Boardworks Ltd 2005 2004

Let’s Recap… There are 3 states of matter that are common to our world. A fourth state, plasma, occurs under conditions not found naturally on earth. Plasma results from high temperatures separating the atom into a sea of charged particles. 1 67 ofof 20 25 © Boardworks Ltd 2005 2004

The 3 states of matter that are commonly observed are solid, liquid, and gas. Water provides a good example of the 3 states. As a solid, we call it ice. As a liquid, we call it water. As a gas, we call it steam or water vapour. 1 68 ofof 20 25 © Boardworks Ltd 2005 2004

Water comes in many different forms: solid – like snow and ice, liquid – like water in a lake, and gas – like steam from a boiling kettle, or water vapour in clouds or fog. These different forms are called states. Can you think of any other substances that you have seen in 3 different states. 1 69 ofof 20 25 © Boardworks Ltd 2005 2004

As a matter of fact, water is the only material that we can normally see on Earth in the form of a solid, liquid, and gas. 1 70 ofof 20 25 © Boardworks Ltd 2005 2004

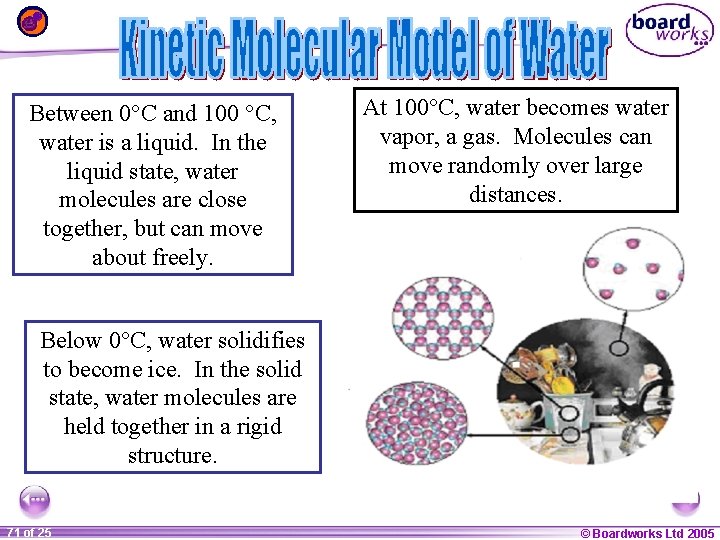
Between 0°C and 100 °C, water is a liquid. In the liquid state, water molecules are close together, but can move about freely. At 100°C, water becomes water vapor, a gas. Molecules can move randomly over large distances. Below 0°C, water solidifies to become ice. In the solid state, water molecules are held together in a rigid structure. 1 71 ofof 20 25 © Boardworks Ltd 2005 2004

Each state of matter – solid, liquid, and gas – has unique properties, or characteristics. A solid is a state of matter that has a definite shape and volume; its shape does not depend on the shape of the container holding it. A liquid is a state of matter that flows, has a fixed volume, and takes the shape of its container. A gas is a state of matter that takes the shape and volume of its container. 1 72 ofof 20 25 © Boardworks Ltd 2005 2004

A change of state occurs when matter changes from one state to another. When a solid changes to a liquid, the melting that occurs is the result of heat energy being added to the solid. The heat energy causes the matter particles to move faster and the bonds between them are broken. The particles are then able to slide around. This is one reason why liquids can take the shape of a container. Addition of more heat energy causes the particles to vibrate even more rapidly. After enough heat energy is added, the particles evaporate into a gas. As a gas cools it condenses into a liquid. Condensation occurs when steam cools, or when heat energy is removed, and water droplets form. Further cooling in a liquid will create a solid. This change of state is called freezing. 1 73 ofof 20 25 © Boardworks Ltd 2005 2004

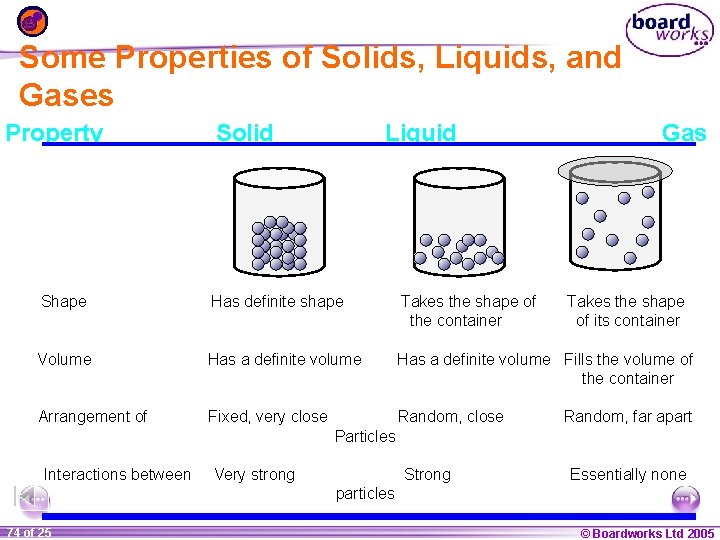
Some Properties of Solids, Liquids, and Gases Property Solid Liquid Gas Shape Has definite shape Takes the shape of the container Takes the shape of its container Volume Has a definite volume Fills the volume of the container Arrangement of Fixed, very close Random, far apart Particles Interactions between Very strong Strong Essentially none particles 1 74 ofof 20 25 © Boardworks Ltd 2005 2004