Thermal Expansion Magnitude of Expansion of Solids Liquids


- Slides: 9

Thermal Expansion Magnitude of Expansion of Solids, Liquids and Gases Examples of Expansion Relationship between pressure and volume at a constant temperature

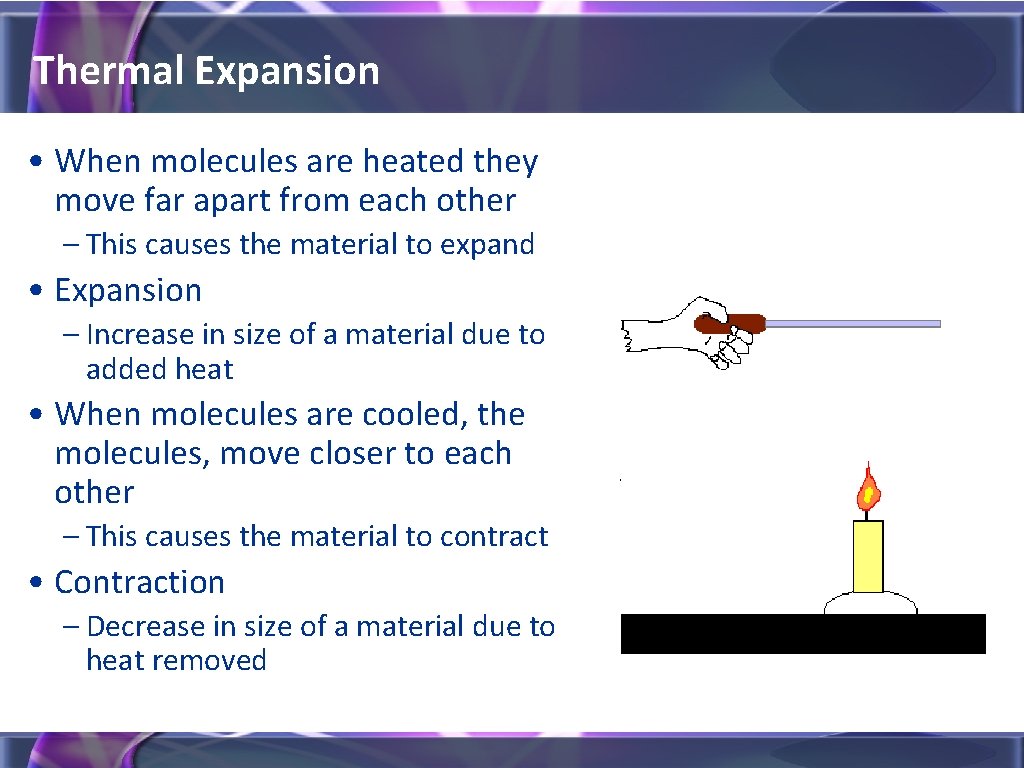
Thermal Expansion • When molecules are heated they move far apart from each other – This causes the material to expand • Expansion – Increase in size of a material due to added heat • When molecules are cooled, the molecules, move closer to each other – This causes the material to contract • Contraction – Decrease in size of a material due to heat removed

Let us look at 3 videos on the example of expansion and contraction

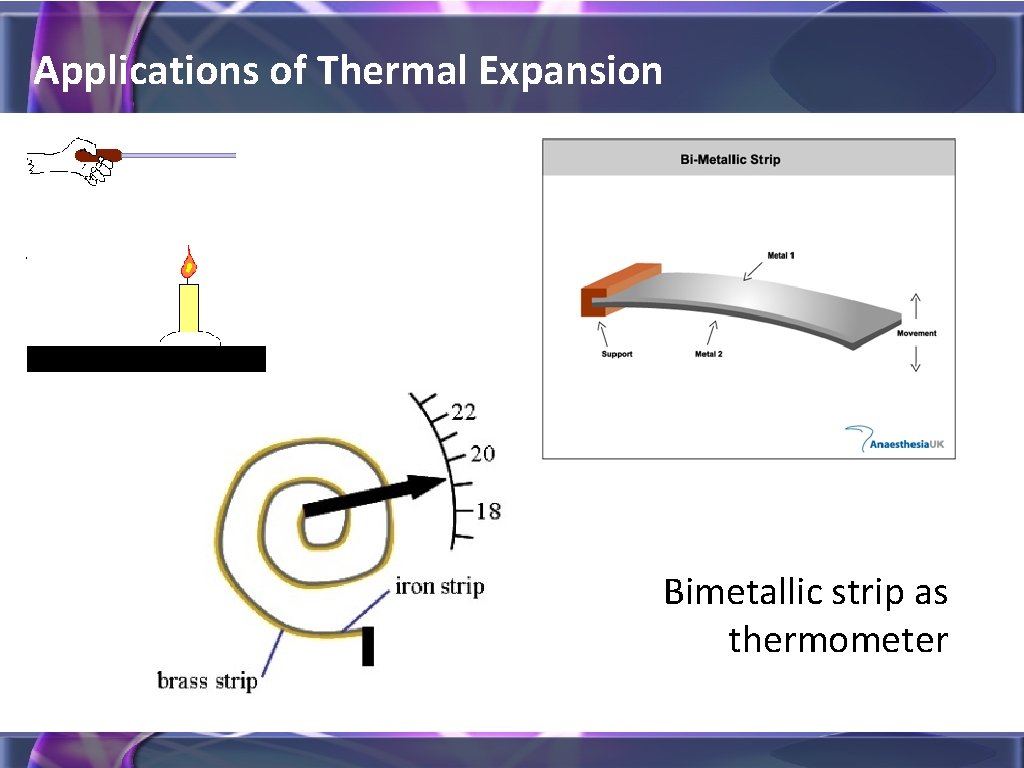
Applications of Thermal Expansion Bimetallic strip as thermometer

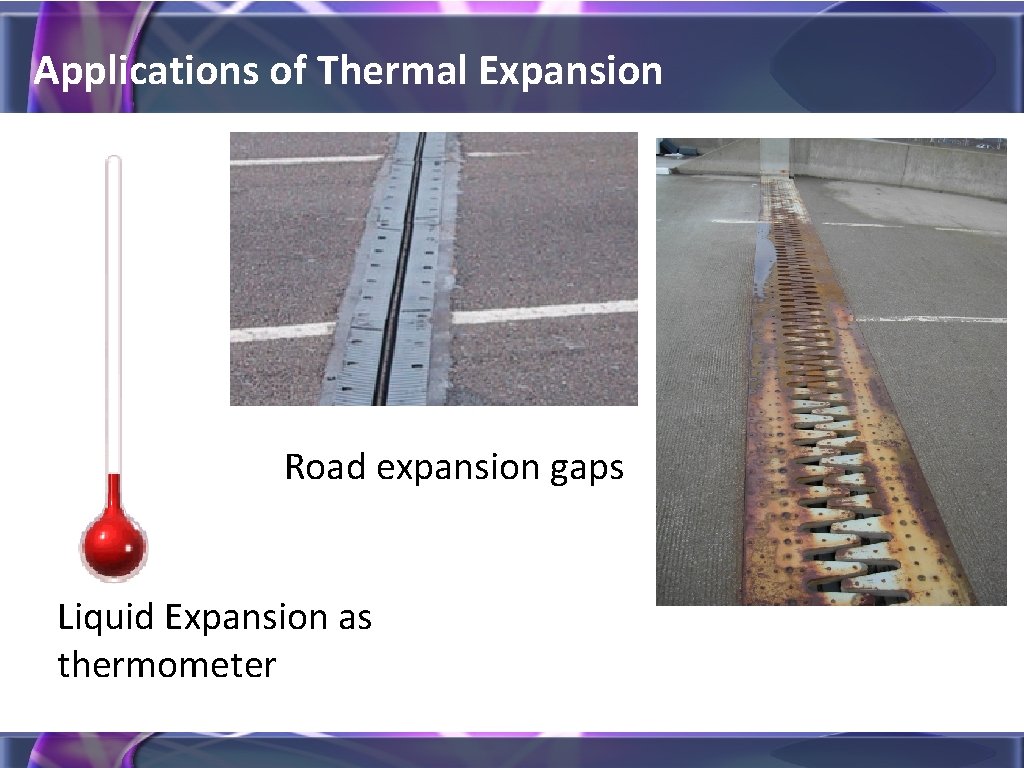
Applications of Thermal Expansion Road expansion gaps Liquid Expansion as thermometer

Applications of Thermal Expansion Tyre Fitting

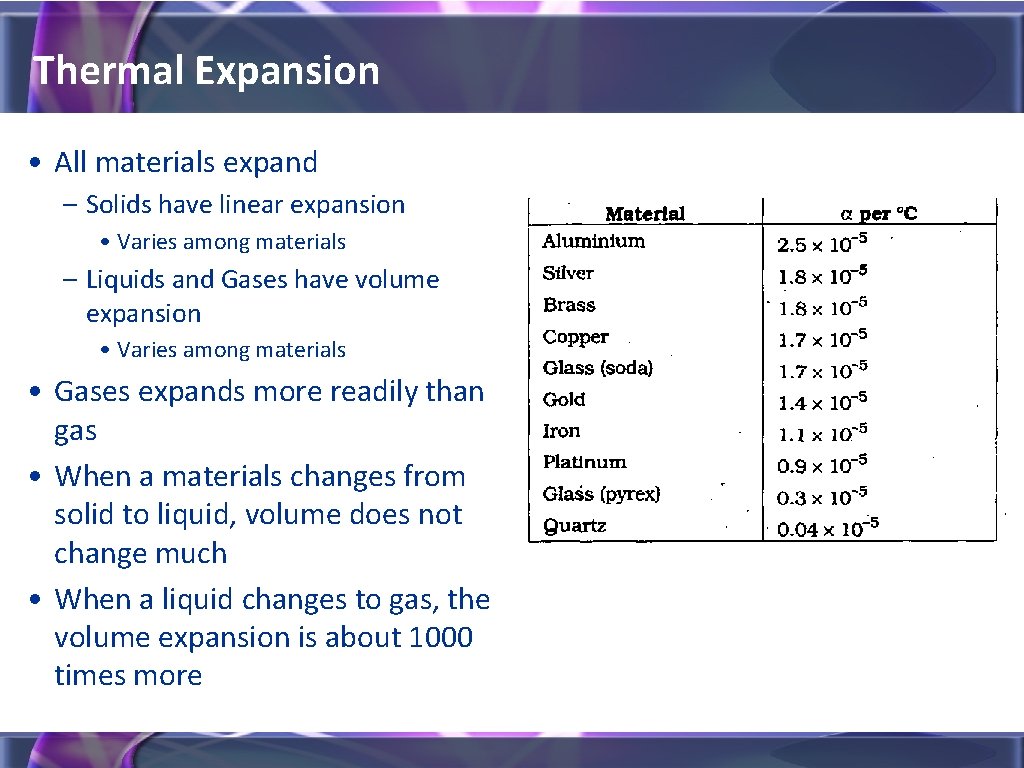
Thermal Expansion • All materials expand – Solids have linear expansion • Varies among materials – Liquids and Gases have volume expansion • Varies among materials • Gases expands more readily than gas • When a materials changes from solid to liquid, volume does not change much • When a liquid changes to gas, the volume expansion is about 1000 times more

Pressure of a Gas • Pressure of a gas – Force per unit area – Force is due to collisions with the walls of the container – Units: Nm-2 or pascal, Pa

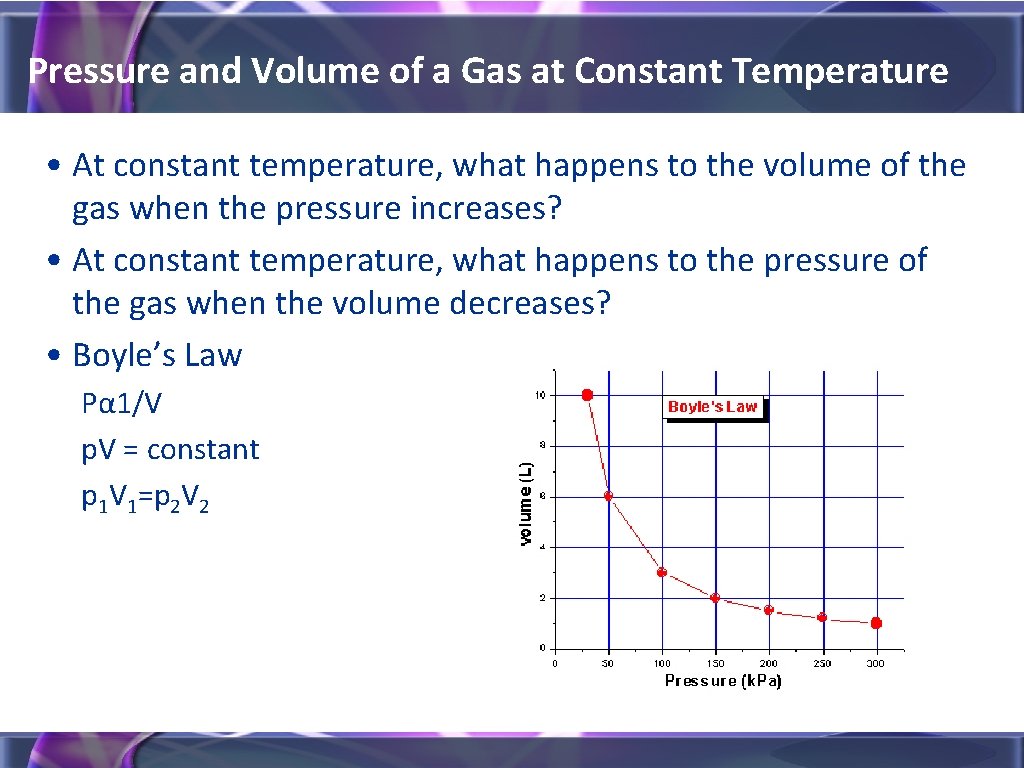
Pressure and Volume of a Gas at Constant Temperature • At constant temperature, what happens to the volume of the gas when the pressure increases? • At constant temperature, what happens to the pressure of the gas when the volume decreases? • Boyle’s Law Pα 1/V p. V = constant p 1 V 1=p 2 V 2
 Expansion of solids liquids and gases examples
Expansion of solids liquids and gases examples Radiate heat
Radiate heat Thermal expansion examples
Thermal expansion examples Thermometer thermal expansion
Thermometer thermal expansion Process of liquid to gas
Process of liquid to gas Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Lesson 1 thermal energy and the behavior of matter
Lesson 1 thermal energy and the behavior of matter Venn diagram of liquid and gas
Venn diagram of liquid and gas Liquid information
Liquid information Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids

















