3 1 Solids Liquids and Gases Solids Liquids










- Slides: 12

3. 1 Solids, Liquids, and Gases

Solids, Liquids, and Gases n The carpenter’s level represents all three states of matter ¨ States of matter: Solids n Liquids n Gases n

Definite Volume or Shape? n Volume-how much space something takes up ¨ An elephant has a greater volume than a pencil n Shape-the way something looks ¨ Think circle of the difference between a square or a

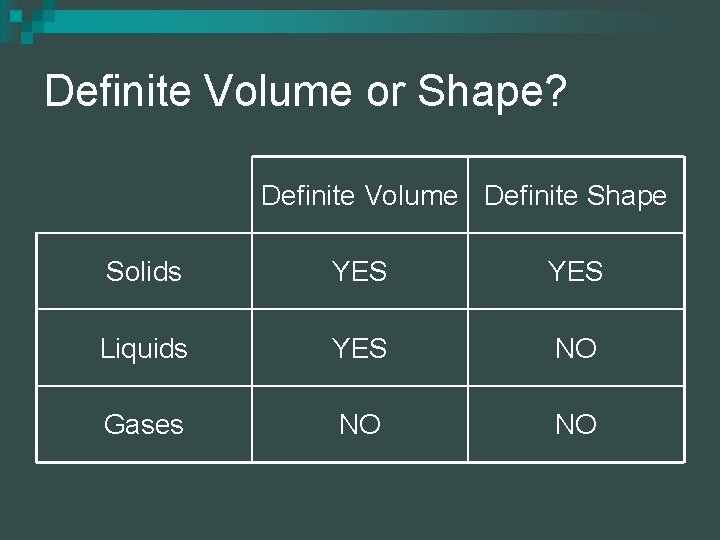
Definite Volume or Shape? Solids: definite volume and definite shape n Liquids: definite volume but no definite shape n Gases: no definite volume or shape n

Definite Volume or Shape? Definite Volume Definite Shape Solids YES Liquids YES NO Gases NO NO

Other States of Matter Almost everything on Earth exists in solid, liquid, or gas form n But nearly everything else in the Universe exists in a state not found on Earth n ¨ Plasma ¨ Bose-Einstein condensate (BEC)

Kinetic Theory Kinetic comes from a Greek word meaning, “to move” n Kinetic energy is the energy an object has due to its motion n ¨ The faster an object moves, the greater its kinetic energy n Ball thrown at 85 mph has more kinetic energy than a ball thrown at 78 mph

Interesting! The batter sees the ball moving n But there is movement within the ball too (that is not seen) n

Kinetic Theory of Matter n This theory simply states that all particles in matter are constantly moving

Explaining Behaviors n Motion in Gases: motion of billiards in a game of pool. ¨ Particles in a gas are in constant, random motion ¨ Motions of particles are unaffected by each other unless they collide ¨ Forces of attraction between particles can be ignored under ordinary conditions

Explaining Behaviors n Motion in Liquids: Like walking through a crowded hallway ¨ Particles are more densely packed ¨ So forces of attraction do affect each other’s motions ¨ Constant tug of war due to motion and attraction

Explaining Behaviors n Motion in solids: like being in a packed movie theatre ¨ Particles vibrate around fixed locations ¨ Particles do not exchange places with their neighboring atoms