Thermal Expansion and contraction Temperature the average amount












- Slides: 18

Thermal Expansion and contraction *Temperature- the average amount of kinetic energy (energy of motion) in an object *Thermal expansion- when an object is heated, molecules move further apart due to their increased kinetic energy. Heated objects EXPAND- volume gets larger

• *Thermal contraction- when an object cools, molecules move closer together due to their decreased kinetic energy. Cooled objects CONTRACT- volume gets smaller • You see this in action when reading a thermometer, adjusting a thermostat, or watching the weather report.

• IMPORTANT: • Solids, liquids and gases all show thermal expansion/contraction, but gases expand/contract the most. • Thermal expansion is a CHARACTERISTIC PROPERTY of solids and liquids- this means solids/liquids expand differently; depending on what material they are made of. •

• All gases expand contract equally, regardless of what they are, so thermal expansion is NOT a characteristic property of gasses. • Charles’ Law : At constant pressure, when temperature increases, volume increases; when temperature decreases, volume decreases. ( direct proportion)

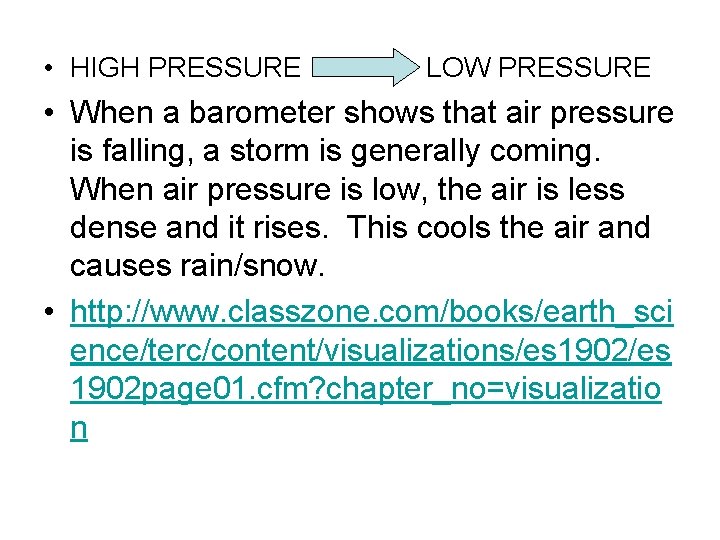
• Thermal contraction/expansion in weather • Air pressure: weight of air molecules • Low air pressure: less air molecules, less dense-warm air • High air pressure: more air molecules, more dense-cold air • High pressure air masses move to areas of low pressure (molecules always move from more crowded areas to less crowded areas)

• HIGH PRESSURE LOW PRESSURE • When a barometer shows that air pressure is falling, a storm is generally coming. When air pressure is low, the air is less dense and it rises. This cools the air and causes rain/snow. • http: //www. classzone. com/books/earth_sci ence/terc/content/visualizations/es 1902 page 01. cfm? chapter_no=visualizatio n

• Thermal Expansion: • When an object ‘s kinetic energy (temperature) increases, the molecules move more rapidly. This causes them to spread apart and the object expands (volume increases). • Thermal Contraction: • When an object‘s kinetic energy (temperature) decreases, the molecules move less rapidly. This causes them to move closer together and the object contracts (volume decreases).

• *Objects expand when heated and shrink when cooled • How does this impact density? • *When an object is heated, its density … • *When an object is cooled, its density…. • Do all states of matter show equal amounts of thermal expansion/contraction? • NO !

• *Solids & Liquids- show LESS thermal expansion and contraction. The amount is specific to each material so it is a CHARACTERISTIC PROPERTY. • *Gases: show the MOST thermal expansion & contraction. All gases expand/contract equally, so it is not a characteristic property for gases.

• SOLIDS: • Thermal expansion is why sidewalks have spaces between sections- so they can expand or contract with changes in temperature.

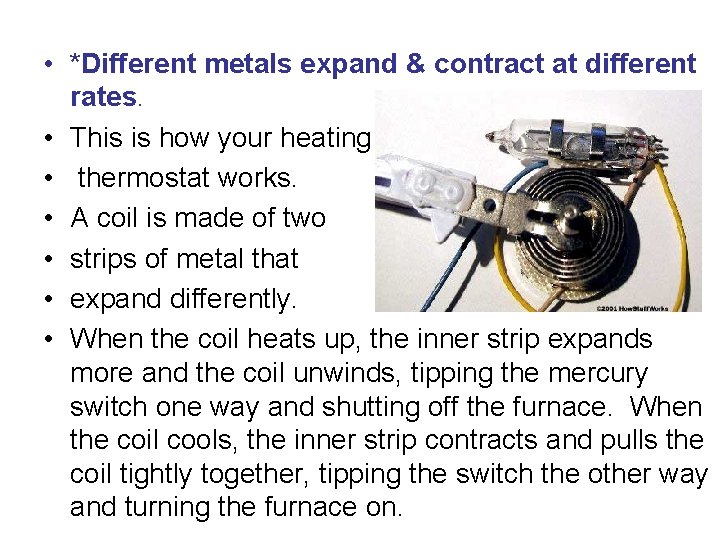
• *Different metals expand & contract at different rates. • This is how your heating • thermostat works. • A coil is made of two • strips of metal that • expand differently. • When the coil heats up, the inner strip expands more and the coil unwinds, tipping the mercury switch one way and shutting off the furnace. When the coil cools, the inner strip contracts and pulls the coil tightly together, tipping the switch the other way and turning the furnace on.

• • • LIQUIDS: Think of an example of how we use thermal expansion of a liquid in an everyday object. * A thermometer works by calibrating the scale on the expansion and contraction of the alcohol in the tube. • Do you think you could fill an alcohol thermometer with mercury and still have it measure temperature accurately?

• A rising environmental concern is the warming of the ocean. If the temperature of the ocean rose 1° C, average sea level would rise about a foot due to thermal expansion. This would move the shore line up about 20 feet!

• GASSES: • Gasses show the greatest thermal expansion and contraction. Why? • We see the concept of gasses and thermal expansion and contraction every night on the news. • Where? • Weather!

*Cold air masses are MORE dense (causing high air pressure) *Warm air masses are LESS dense (causing low air pressure). When a cold air mass meets a warm air masswhat do you think happens? http: //www. classzone. com/books/earth_science/ terc/content/visualizations/es 2002 page 0 1. cfm? chapter_no=visualization

Conclusion Using your notes, explain in detail why: 1. the egg entered the bottle 2. the cap entered the flask 3. the can became crushed. Give examples

• If……then…. . because • If there is a change in pressure then : – The can will crush – The egg will enter the bottle because:

• How does it work? • Here’s the real scoop on the science of the imploding can. Before heating, the can was filled with water and air. By boiling the water, the water changed states from a liquid to a gas. This gas is called water vapor. The water vapor pushed the air that was originally inside the can out into the atmosphere. When the can was turned upside down and placed in the water, the water vapor condensed and turned back into the water. Water molecules in the liquid state are many times closer together than molecules in the gas state. All of the water vapor that filled up the inside of the can turned into only a drop or two of liquid, which took up much less space. • This small amount of water cannot exert much pressure on the inside walls of the can, so the pressure of the air pushing from the outside of the can is great enough to crush it. The sudden collapsing of an object toward its center is called an implosion. Nature wants things to be in a state of equilibrium or balance. To make the internal pressure of the can balance with the external pressure on the can, the can implodes. Hey, air pressure is powerful!