GASES LIQUIDS AND SOLIDS Comparison of solids liquids












- Slides: 27

GASES, LIQUIDS AND SOLIDS

Comparison of solids, liquids and gases: Gas ■ Assumes the shape and volume of its container ■ Is compressible ■ Flows readily; diffusion within a gas occurs rapidly Liquid ■ Assumes the shape of the portion of the container it occupies; does not expand to fill its container ■ Is virtually incompressible ■ Flows readily; diffusion within a liquid occurs slowly Solid ■ Retains its own shape and volume ■ Is virtually incompressible ■ Does not flow ■ Diffusion within a solid occurs extremely slowly

Solids

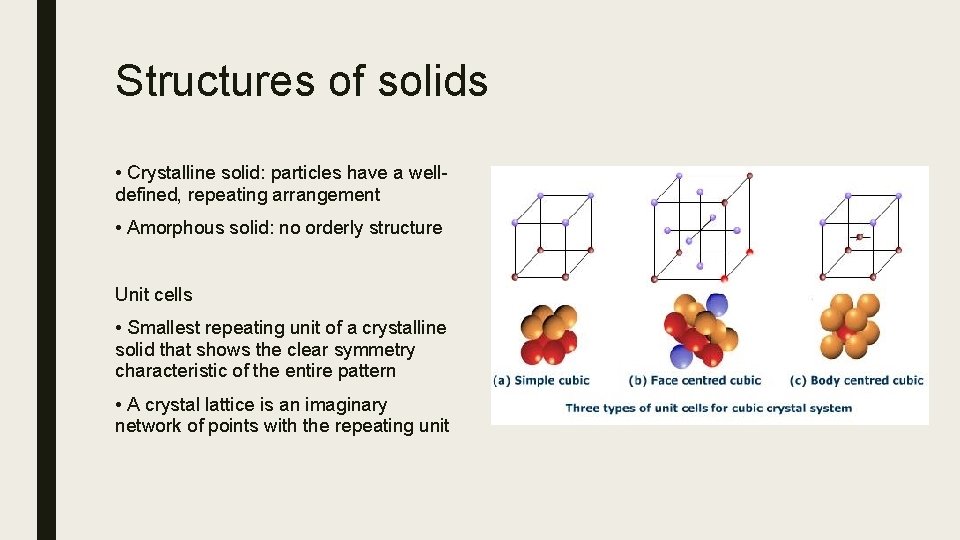
Structures of solids • Crystalline solid: particles have a welldefined, repeating arrangement • Amorphous solid: no orderly structure Unit cells • Smallest repeating unit of a crystalline solid that shows the clear symmetry characteristic of the entire pattern • A crystal lattice is an imaginary network of points with the repeating unit

Types of Solids

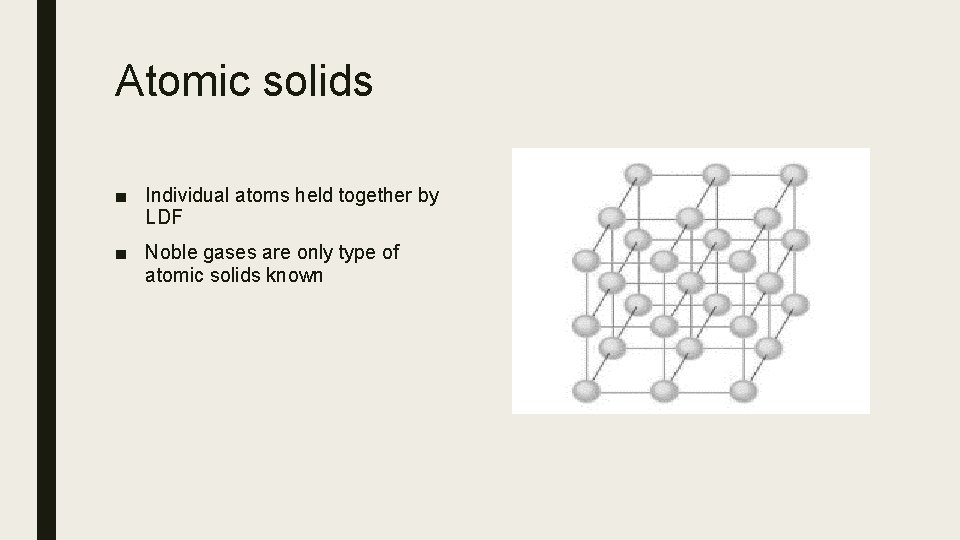
Atomic solids ■ Individual atoms held together by LDF ■ Noble gases are only type of atomic solids known

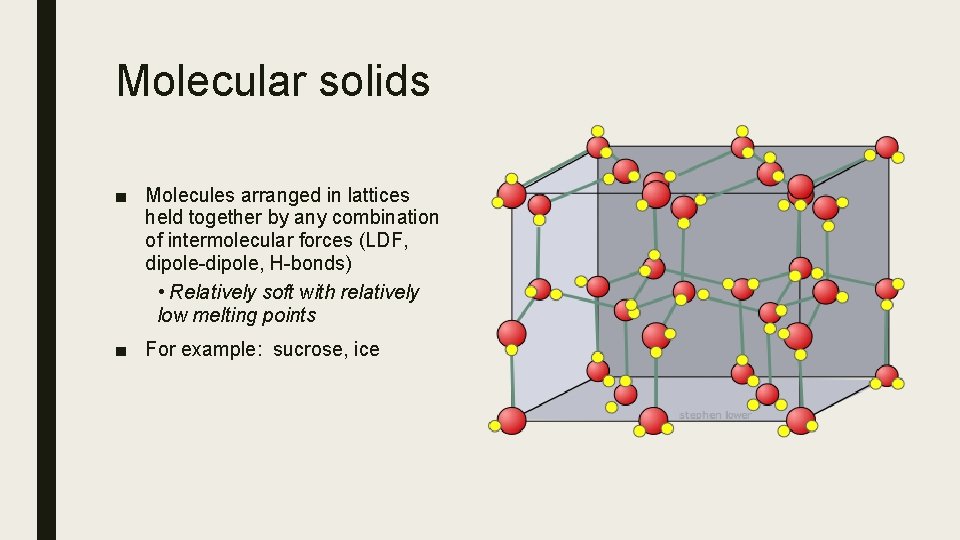
Molecular solids ■ Molecules arranged in lattices held together by any combination of intermolecular forces (LDF, dipole-dipole, H-bonds) • Relatively soft with relatively low melting points ■ For example: sucrose, ice

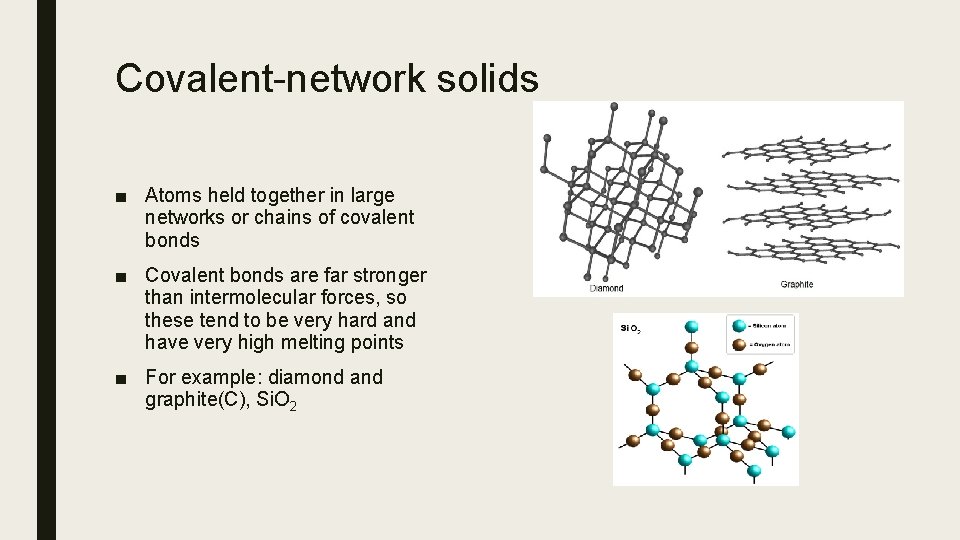
Covalent-network solids ■ Atoms held together in large networks or chains of covalent bonds ■ Covalent bonds are far stronger than intermolecular forces, so these tend to be very hard and have very high melting points ■ For example: diamond and graphite(C), Si. O 2

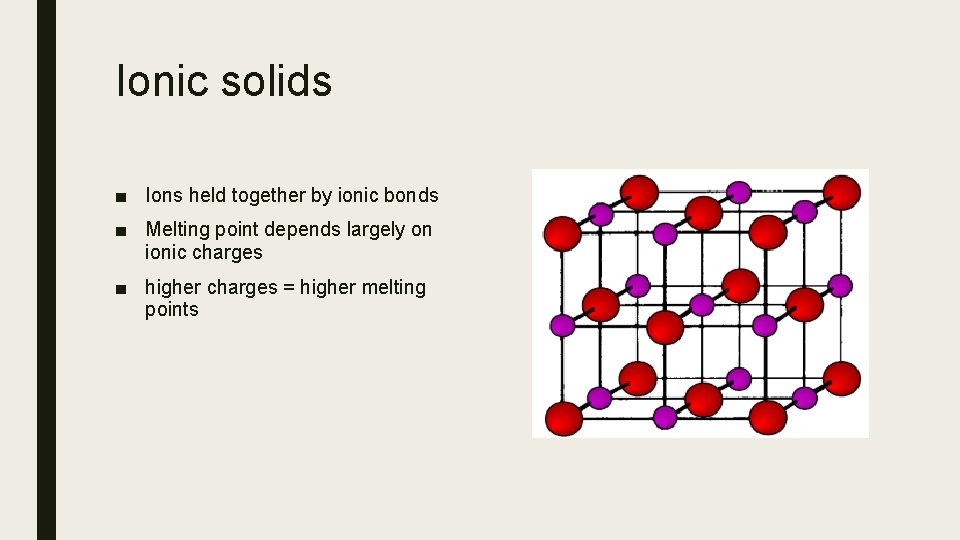
Ionic solids ■ Ions held together by ionic bonds ■ Melting point depends largely on ionic charges ■ higher charges = higher melting points

Metallic solids ■ Metal ions held together by valence electrons that are delocalized throughout the solid ■ Melting points may be relatively high OR low, but boiling points are always very high ■ Delocalized electrons explain why metals are good conductors of heat and electricity

■ AP practice question

Some Properties of Liquids

Viscosity the resistance of a liquid to flow ■ Stronger intermolecular forces = higher viscosity ■ Higher temperature = lower viscosity ■ Molecules have more kinetic energy ■ More easily overcome attractive forces (IMF)

Surface tension ■ Molecules on the surface of a liquid experience a net inward force ■ Due to increased inward force, surface molecules pack together more tightly than interior molecules

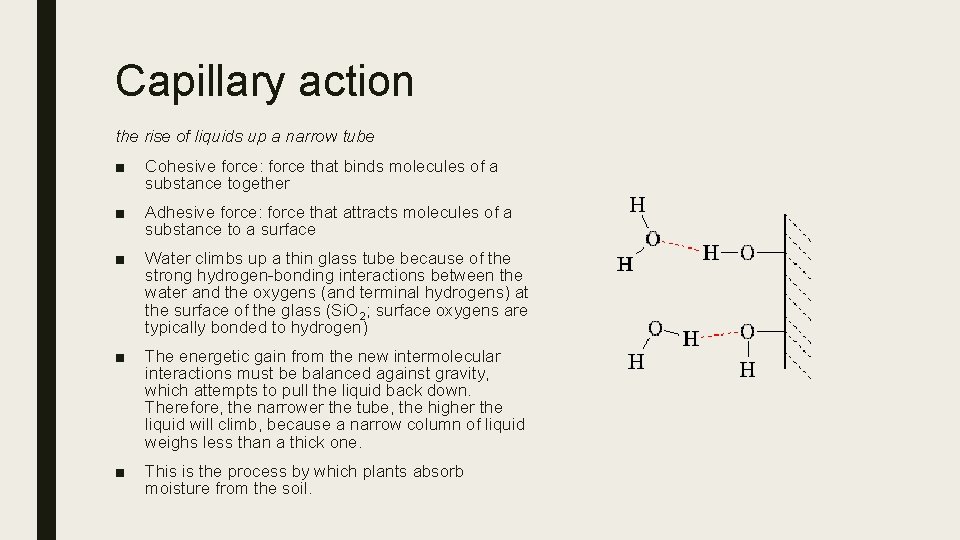
Capillary action the rise of liquids up a narrow tube ■ Cohesive force: force that binds molecules of a substance together ■ Adhesive force: force that attracts molecules of a substance to a surface ■ Water climbs up a thin glass tube because of the strong hydrogen-bonding interactions between the water and the oxygens (and terminal hydrogens) at the surface of the glass (Si. O 2; surface oxygens are typically bonded to hydrogen) ■ The energetic gain from the new intermolecular interactions must be balanced against gravity, which attempts to pull the liquid back down. Therefore, the narrower the tube, the higher the liquid will climb, because a narrow column of liquid weighs less than a thick one. ■ This is the process by which plants absorb moisture from the soil.

Properties of Gases

Vapor Pressure: the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample of the liquid (or solid) in a closed container.

Explaining vapor pressure on the molecular level: ■ Liquid molecules move at various speeds ■ Molecules at the surface of the liquid possessing sufficient kinetic energy will escape ■ Molecules in the gas phase striking the surface of the liquid will be recaptured ■ Weaker intermolecular forces = more molecules possess minimum energy to escape = higher vapor pressure ■ Molecules are constantly escaping and different molecules are being recaptured at an equal rate ■ Increase in temperature results in more molecules having the minimum energy required to escape, therefore vapor pressure increases until an equal rate of escape and recapture (equilibrium) is reestablished ■ Vapor pressure is the pressure exerted by the vapor of a liquid when the liquid and vapor states are at equilibrium

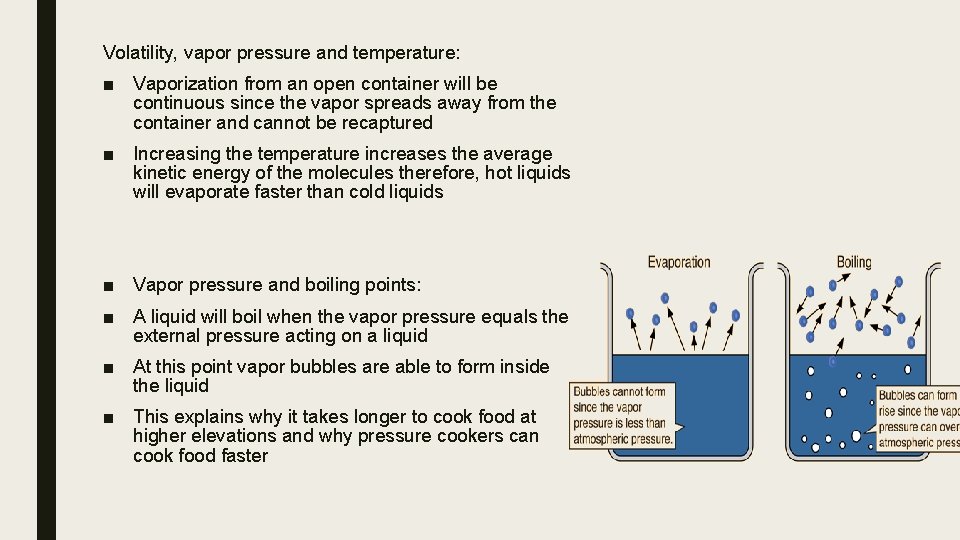
Volatility, vapor pressure and temperature: ■ Vaporization from an open container will be continuous since the vapor spreads away from the container and cannot be recaptured ■ Increasing the temperature increases the average kinetic energy of the molecules therefore, hot liquids will evaporate faster than cold liquids ■ Vapor pressure and boiling points: ■ A liquid will boil when the vapor pressure equals the external pressure acting on a liquid ■ At this point vapor bubbles are able to form inside the liquid ■ This explains why it takes longer to cook food at higher elevations and why pressure cookers can cook food faster

■ AP practice questions

Ideal Gas Law ■ AP practice questions

Kinetic Molecular Theory The following are the basic assumptions of the Kinetic Molecular Theory: ■ The volume occupied by the individual particles of a gas is negligible compared to the volume of the gas itself. ■ The particles of an ideal gas exert no attractive forces on each other or on their surroundings. ■ Gas particles are in a constant state of random motion, referred to as Brownian motion, and move in straight lines until they collide with another body. ■ The collisions exhibited by gas particles are completely elastic; when two molecules collide, total kinetic energy is conserved. ■ The average kinetic energy of gas molecules is directly proportional to absolute temperature only; this implies that all molecular motion ceases if the temperature is reduced to absolute zero.

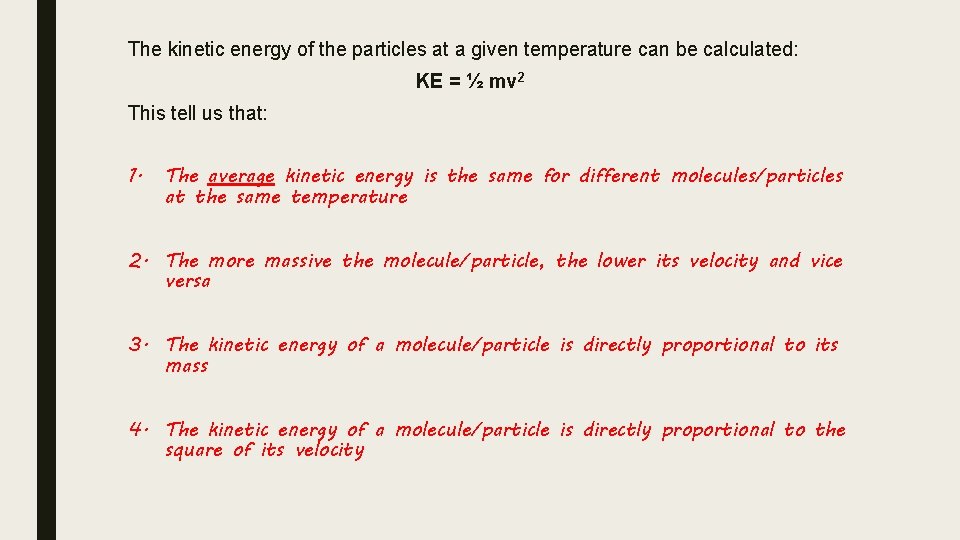
The kinetic energy of the particles at a given temperature can be calculated: KE = ½ mv 2 This tell us that: 1. The average kinetic energy is the same for different molecules/particles at the same temperature 2. The more massive the molecule/particle, the lower its velocity and vice versa 3. The kinetic energy of a molecule/particle is directly proportional to its mass 4. The kinetic energy of a molecule/particle is directly proportional to the square of its velocity

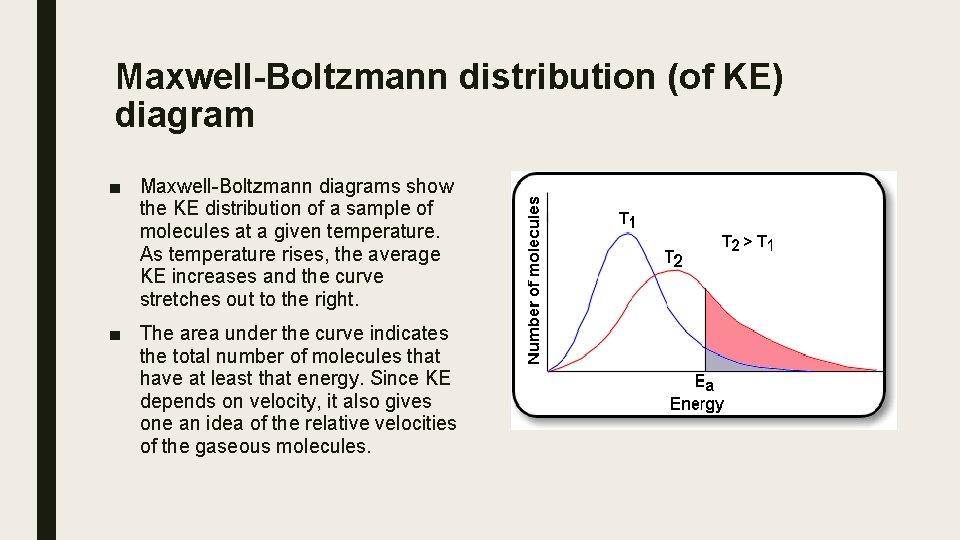
Maxwell-Boltzmann distribution (of KE) diagram ■ Maxwell-Boltzmann diagrams show the KE distribution of a sample of molecules at a given temperature. As temperature rises, the average KE increases and the curve stretches out to the right. ■ The area under the curve indicates the total number of molecules that have at least that energy. Since KE depends on velocity, it also gives one an idea of the relative velocities of the gaseous molecules.

■ AP practice question

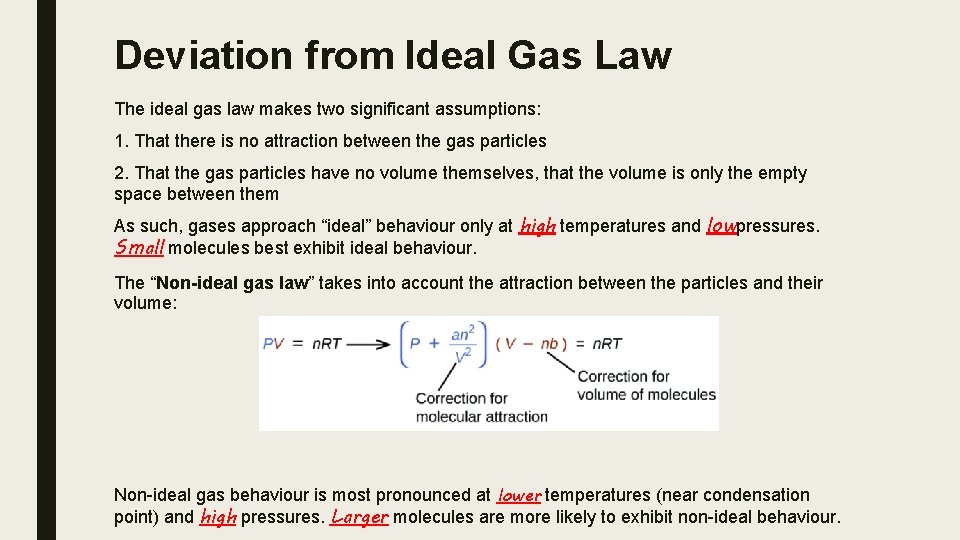
Deviation from Ideal Gas Law The ideal gas law makes two significant assumptions: 1. That there is no attraction between the gas particles 2. That the gas particles have no volume themselves, that the volume is only the empty space between them As such, gases approach “ideal” behaviour only at high temperatures and lowpressures. Small molecules best exhibit ideal behaviour. The “Non-ideal gas law” takes into account the attraction between the particles and their volume: Non-ideal gas behaviour is most pronounced at lower temperatures (near condensation point) and high pressures. Larger molecules are more likely to exhibit non-ideal behaviour.

■ AP practice question