CHAPTER 13 GASES intro Date Solids Liquids Gases

- Slides: 8

CHAPTER 13 GASES (intro) Date________ Solids Liquids Gases

CHAPTER 13 GASES (intro) Date________ Solids Liquids Gases

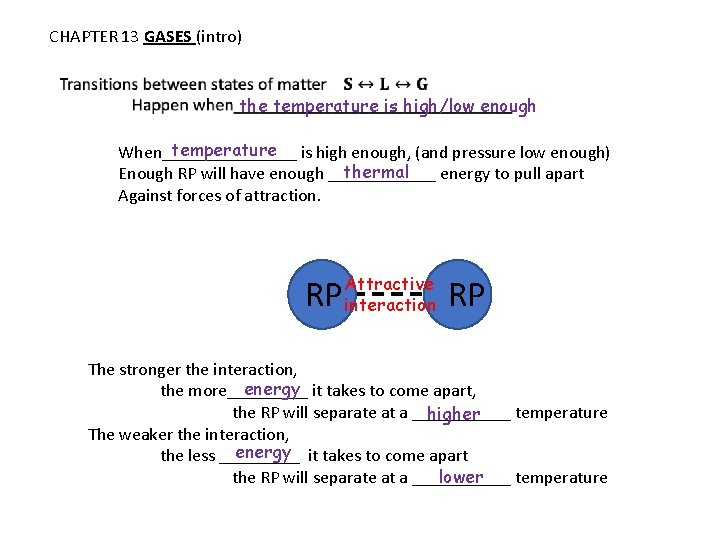
CHAPTER 13 GASES (intro) the temperature is high/low enough temperature is high enough, (and pressure low enough) When________ thermal Enough RP will have enough ______ energy to pull apart Against forces of attraction. RP Attractive interaction RP The stronger the interaction, energy it takes to come apart, the more_____ the RP will separate at a ______ temperature higher The weaker the interaction, energy it takes to come apart the less _____ the RP will separate at a ______ temperature lower

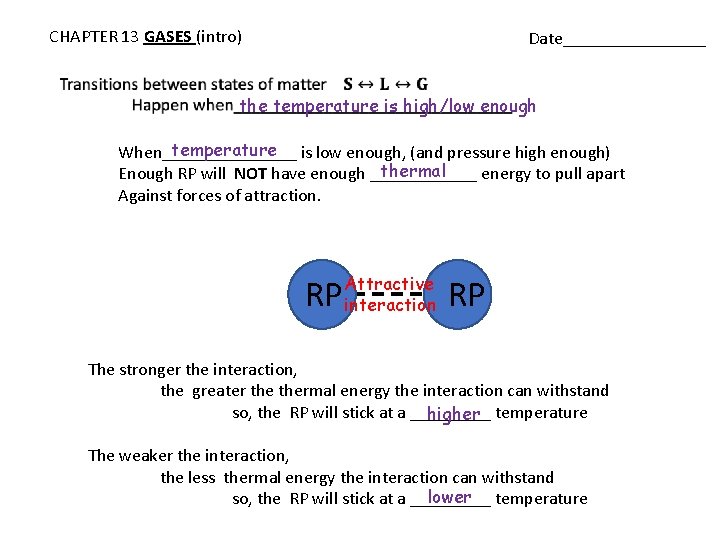
CHAPTER 13 GASES (intro) Date________ the temperature is high/low enough temperature is low enough, (and pressure high enough) When________ thermal Enough RP will NOT have enough ______ energy to pull apart Against forces of attraction. RP Attractive interaction RP The stronger the interaction, the greater thermal energy the interaction can withstand so, the RP will stick at a _____ higher temperature The weaker the interaction, the less thermal energy the interaction can withstand lower temperature so, the RP will stick at a _____

A case for Nitrogen (N 2) The interactions between the RP’s of N 2 are…… strong / weak It takes a small amount / a large amount of thermal energy to separate the particles The boiling point of N 2 is -195. 8℃ …. . Interactions between N 2 molecules are so weak that the molecules can escape one another with very little thermal energy. ty es p lv em e. The number of RP’s in our sample of N 2 stayed the same as stly emse spac mo ’s th ittle it boiled…… e ar e RP ly l s se ! Th ative a G ce Yet the volume of our sampled increased. el r a sp e up tak

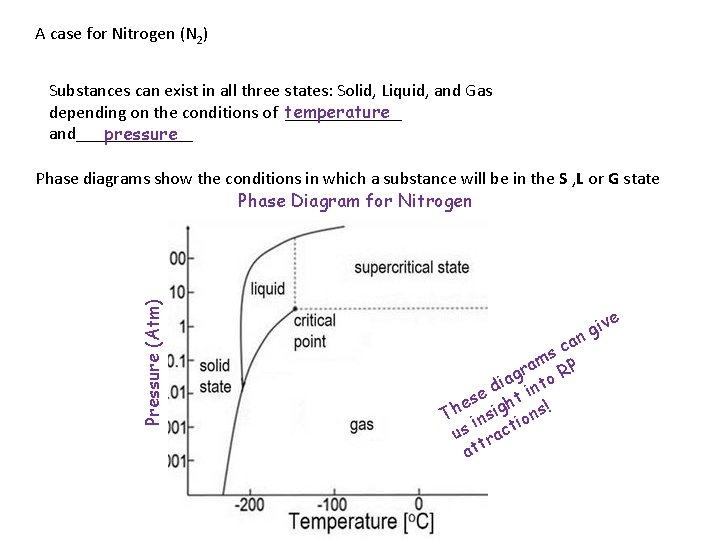
A case for Nitrogen (N 2) Substances can exist in all three states: Solid, Liquid, and Gas depending on the conditions of temperature _______ and_______ pressure Pressure (Atm) Phase diagrams show the conditions in which a substance will be in the S , L or G state Phase Diagram for Nitrogen ive g an c s m a r RP g a o di int e es ight s! h T ins ion us ract att

RP RP We will start with the behavior of gases (substances in the gas state) RP They’re the easiest to describe, and explain Because……. RP The RP are “all by themselves” -separated from each other The RP behave (nearly Independently) RP RP

Have to define an Ideal gas Since there is (relatively) a lot of space between RP in the gas state, one can approximate that there are NO interactions between RP No attraction between RP No repulsions between RP RP can collide, and bounce back with the same energy To recognize that this would be an “ideal gas” …consider. . what would happen as the temp. of the gas decreased? _______________________ The RP would start to slow down eventually (if it got cold enough) The gas would turn into a liquid _______________________ STUCK which means the RP are ______ together Thus they ARE attracting and, NOT ideal As we go through the behaviors of gases, we will assume that they are all ideal