Chapter Introduction Lesson 1 Solids Liquids and Gases














- Slides: 22

Chapter Introduction Lesson 1 Solids, Liquids, and Gases Lesson 2 Changes in State Lesson 3 The Behavior of Gases Chapter Wrap-Up

Do you agree or disagree? Sticky Note Post 1. Particles moving at the same speed make up all matter. 2. The particles in a solid do not move. 3. Particles of matter have both potential energy and kinetic energy. 4. When a solid melts, thermal energy is removed from the solid. 5. Changes in temperature and pressure affect gas behavior 6. If the pressure on a gas increases, the volume of the gas also increases.

Describing Matter (cont. ) 1. In some matter, the particles move slowly. 2. The particles vibrate in place. 1. In other matter, the particles move faster and slip past each other. 3. The attractive forces between the particle are strong. 2. The distance between the particles increases. 3. The attractive forces between the particles are weaker.

Describing Matter (cont. ) 1. In other matter, the particles move very fast. 2. The distance between the particles is great. 3. The attractive forces between the particles are very weak.

Describing Matter (cont. ) 1. As the motion of the particles slows, the particles move closer. The attractive forces become stronger. 2. As the motion of the particles increases, the particles move farther apart. The attractive forces become weaker.

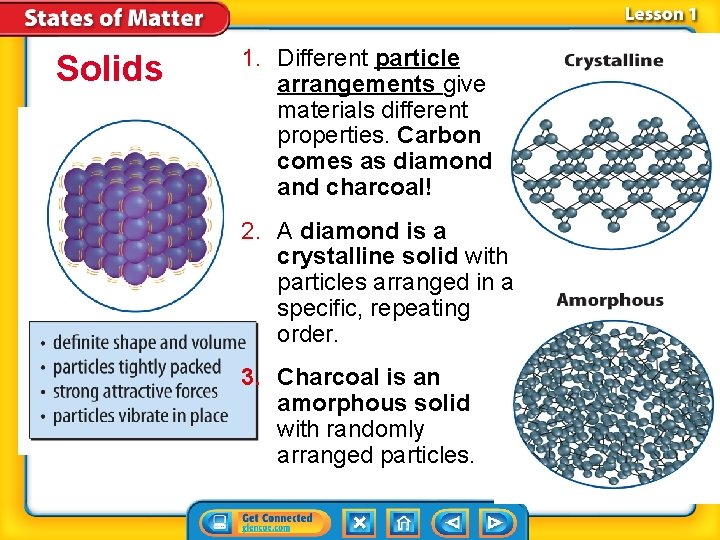
Solids 1. Different particle arrangements give materials different properties. Carbon comes as diamond and charcoal! 2. A diamond is a crystalline solid with particles arranged in a specific, repeating order. 3. Charcoal is an amorphous solid with randomly arranged particles.

Liquids 1. Viscosity is a measurement of a liquid’s resistance to flow.

Liquids (cont. ) 1. Molecules at the surface of a liquid have surface tension, the uneven forces acting on the particles on the surface of a liquid. 2. Get a small cup and water, try floating a paperclip on top of the water.

Gases 1. In a gas, the forces of attraction between the particles are not strong enough to keep the particles close together. 2. The gas state of a substance that is normally a solid or a liquid at room temperature is called vapor.

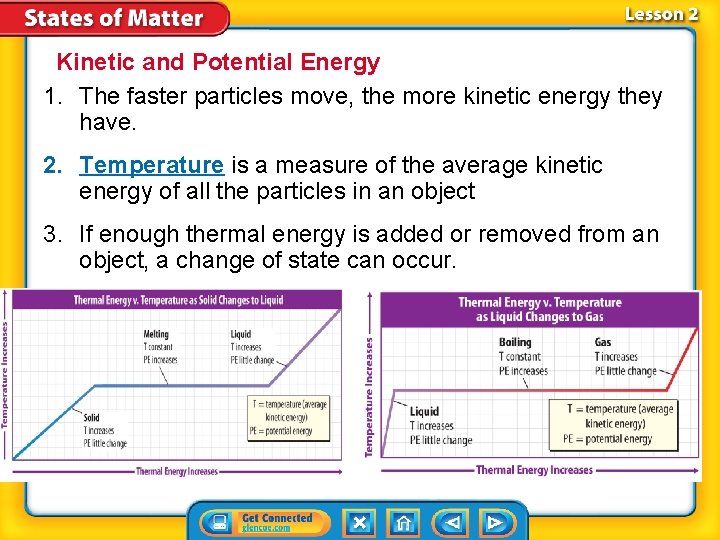
Kinetic and Potential Energy 1. The faster particles move, the more kinetic energy they have. 2. Temperature is a measure of the average kinetic energy of all the particles in an object 3. If enough thermal energy is added or removed from an object, a change of state can occur.

Solid to Gas or Gas to Solid 1. Sublimation is the change of state from a solid to a gas without going through the liquid state. 2. Deposition is the change of state of a gas to a solid without going through the liquid state.

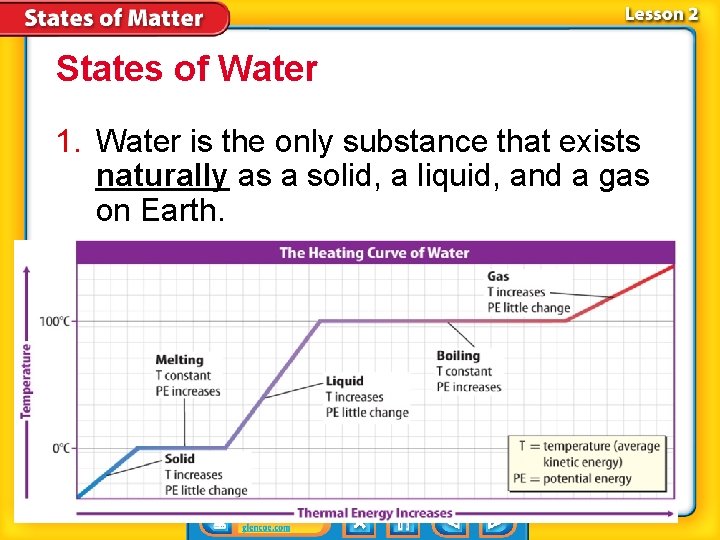
States of Water 1. Water is the only substance that exists naturally as a solid, a liquid, and a gas on Earth.

Understanding Gas Behavior The kinetic molecular theory states that the particles in matter collide with other particles, other objects, and the walls of their container; and when particles collide, no energy is lost.

What is pressure? • Pressure is the amount of force applied per unit of area. • The empty spaces between particles makes gases compressible.

Pressure and Volume When the volume of a container holding gas is greater, the additional space results in fewer collisions and pressure is less.

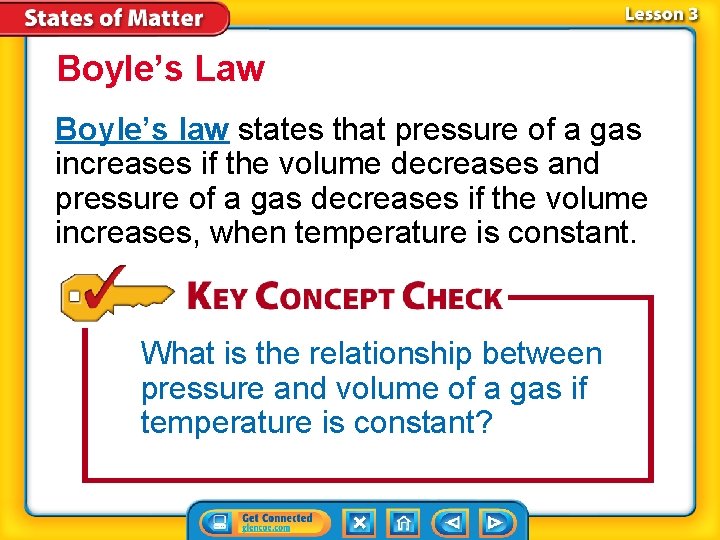
Boyle’s Law Boyle’s law states that pressure of a gas increases if the volume decreases and pressure of a gas decreases if the volume increases, when temperature is constant. What is the relationship between pressure and volume of a gas if temperature is constant?

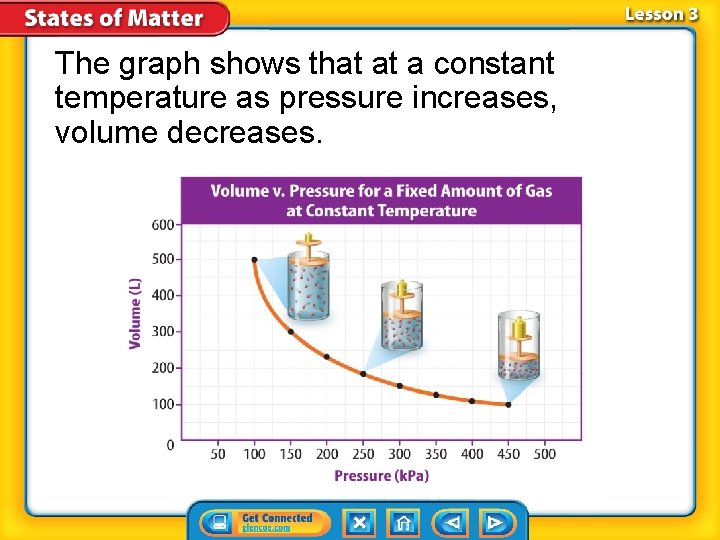
The graph shows that at a constant temperature as pressure increases, volume decreases.

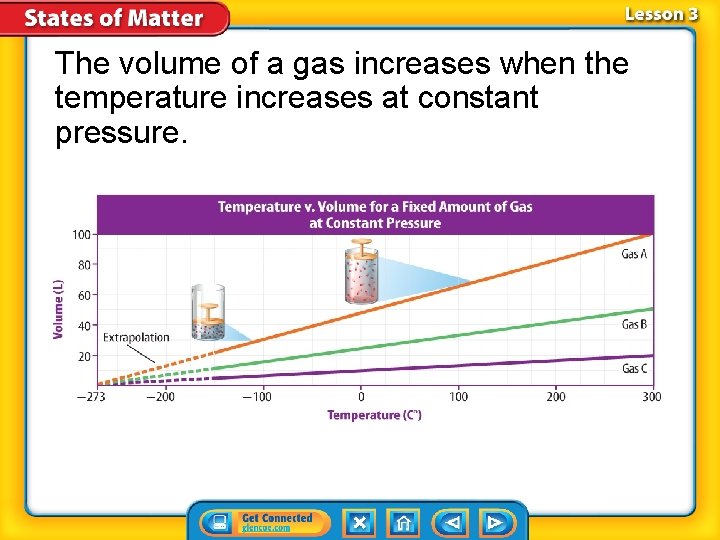
Temperature and Volume • Changing the temperature of a gas affects its behavior. • As the temperature of a gas increases, kinetic energy increases, the particles move farther apart, and volume increases.

Temperature and Volume (cont. )

Charles’s Law Charles’s law states that the volume of a gas increases with increasing temperature, if the pressure is constant. How is Boyle’s law different from Charles’s law?

The volume of a gas increases when the temperature increases at constant pressure.

Charles’s Law (cont. ) What factors must be constant in Boyle’s law and Charles’s law?