CRCT Review Physical Science Mrs Gerrin Standard 1



























- Slides: 62

CRCT Review Physical Science Mrs. Gerrin

Standard 1 Students will examine the scientific view of the nature of matter.

Matter • Matter is anything with mass and volume.

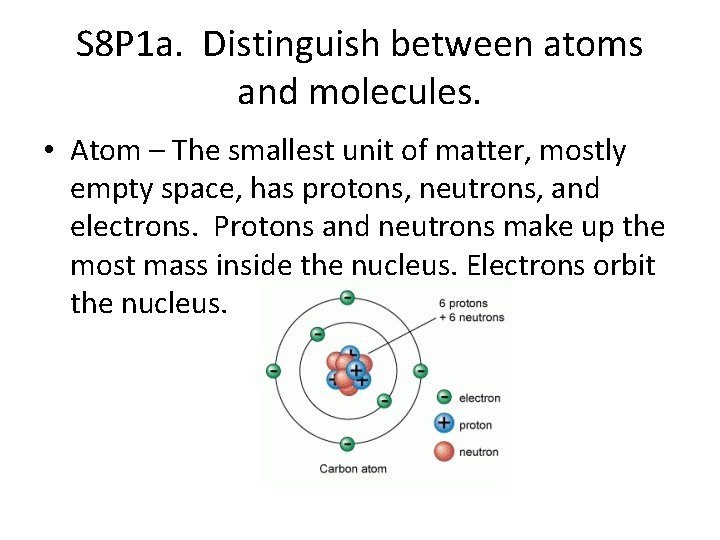
S 8 P 1 a. Distinguish between atoms and molecules. • Atom – The smallest unit of matter, mostly empty space, has protons, neutrons, and electrons. Protons and neutrons make up the most mass inside the nucleus. Electrons orbit the nucleus.

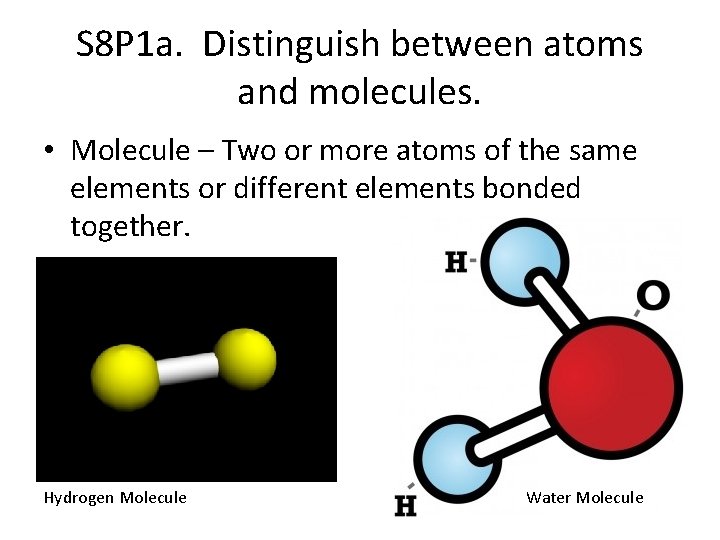
S 8 P 1 a. Distinguish between atoms and molecules. • Molecule – Two or more atoms of the same elements or different elements bonded together. Hydrogen Molecule Water Molecule

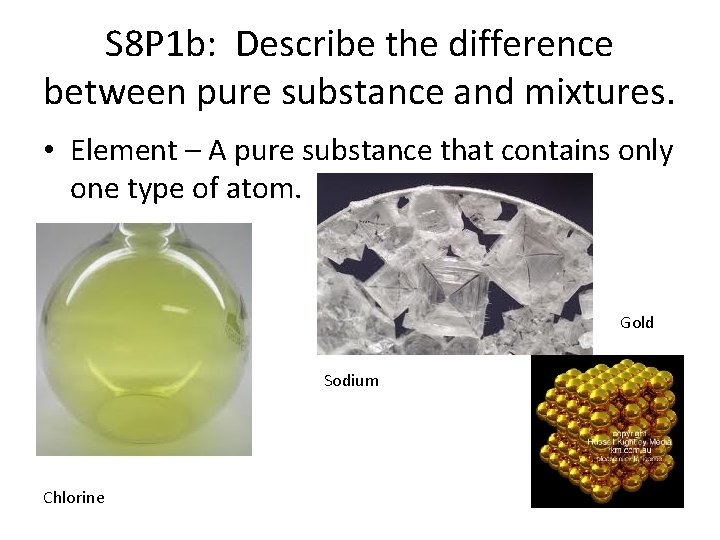
S 8 P 1 b: Describe the difference between pure substance and mixtures. • Element – A pure substance that contains only one type of atom. Gold Sodium Chlorine

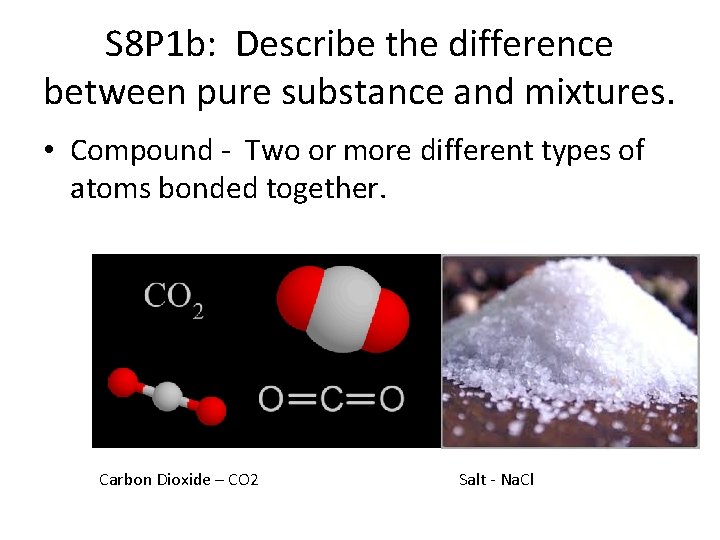
S 8 P 1 b: Describe the difference between pure substance and mixtures. • Compound - Two or more different types of atoms bonded together. Carbon Dioxide – CO 2 Salt - Na. Cl

S 8 P 1 b: Describe the difference between pure substance and mixtures. • Mixtures – a combination of different substances that remain the same and can be separated by physical means. • Heterogeneous – different substances in different areas of the mixture, ex. Soil, trail mix, salad. • Homogeneous – substances are spread evenly throughout, ex. Milk, sweet tea, lemonade.

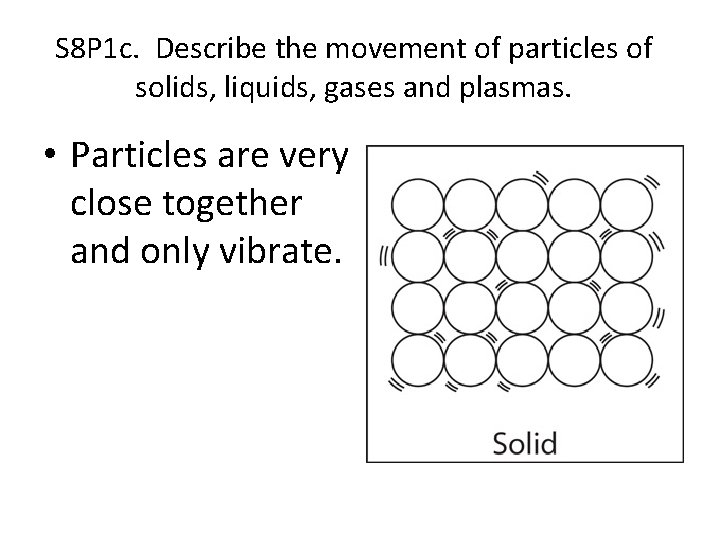
S 8 P 1 c. Describe the movement of particles of solids, liquids, gases and plasmas. • Particles are very close together and only vibrate.

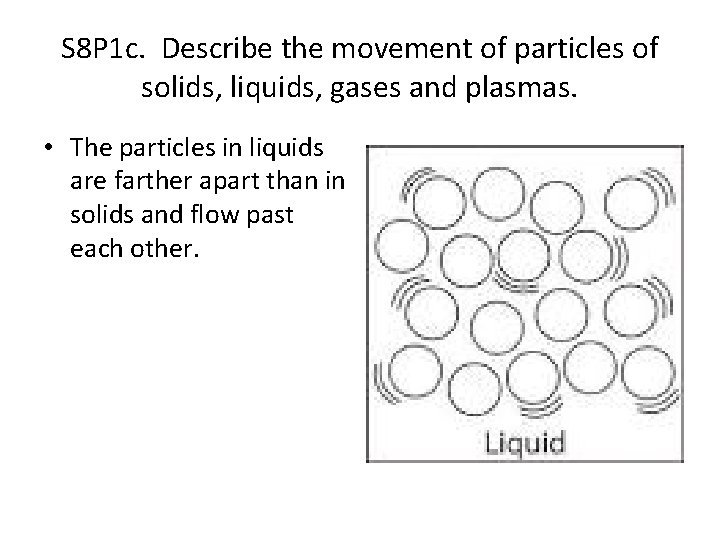
S 8 P 1 c. Describe the movement of particles of solids, liquids, gases and plasmas. • The particles in liquids are farther apart than in solids and flow past each other.

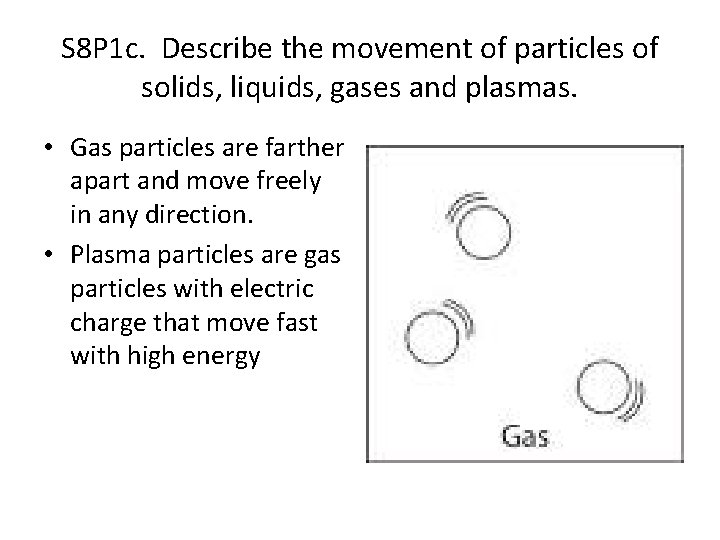
S 8 P 1 c. Describe the movement of particles of solids, liquids, gases and plasmas. • Gas particles are farther apart and move freely in any direction. • Plasma particles are gas particles with electric charge that move fast with high energy

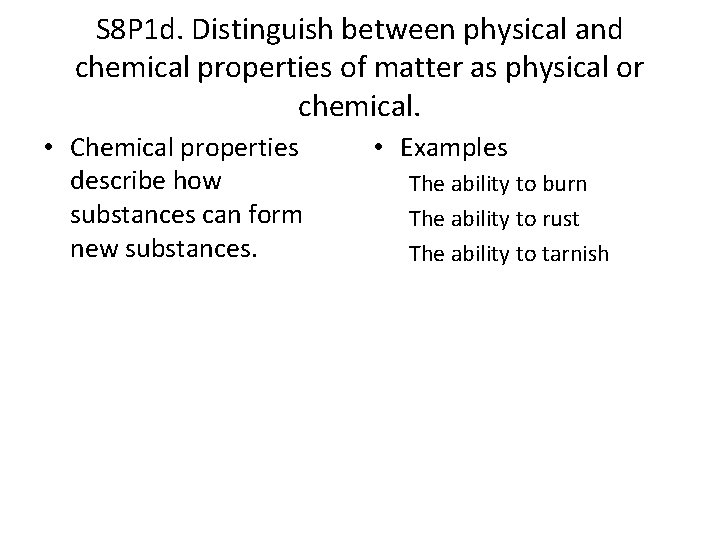
S 8 P 1 d. Distinguish between physical and chemical properties of matter as physical or chemical. • Physical Properties are characteristics of a substance that can be observed without changing the identity of the substance. • Examples: color size shape density melting point volume state of matter

S 8 P 1 d. Distinguish between physical and chemical properties of matter as physical or chemical. • Chemical properties describe how substances can form new substances. • Examples The ability to burn The ability to rust The ability to tarnish

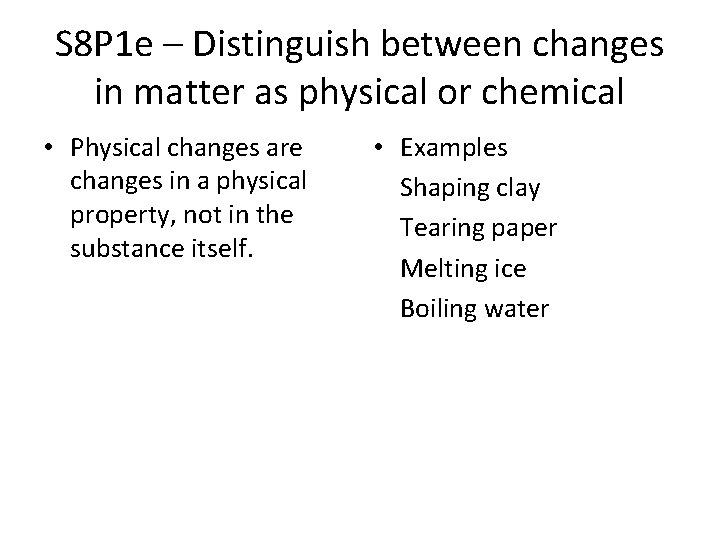
S 8 P 1 e – Distinguish between changes in matter as physical or chemical • Physical changes are changes in a physical property, not in the substance itself. • Examples Shaping clay Tearing paper Melting ice Boiling water

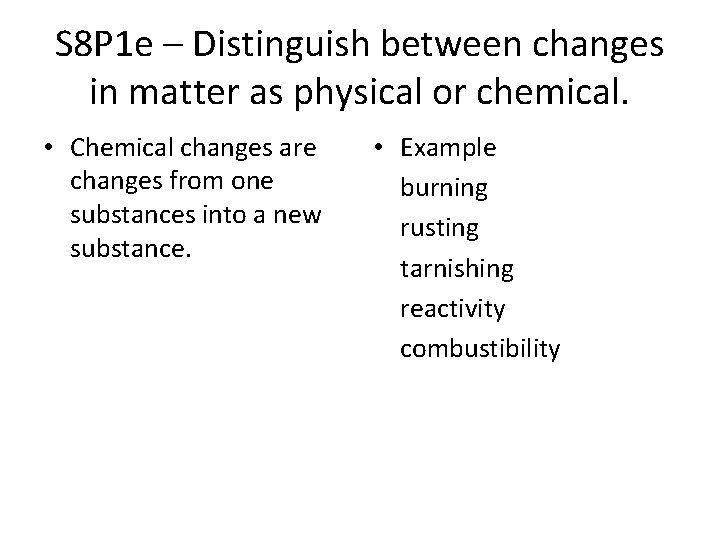
S 8 P 1 e – Distinguish between changes in matter as physical or chemical. • Chemical changes are changes from one substances into a new substance. • Example burning rusting tarnishing reactivity combustibility

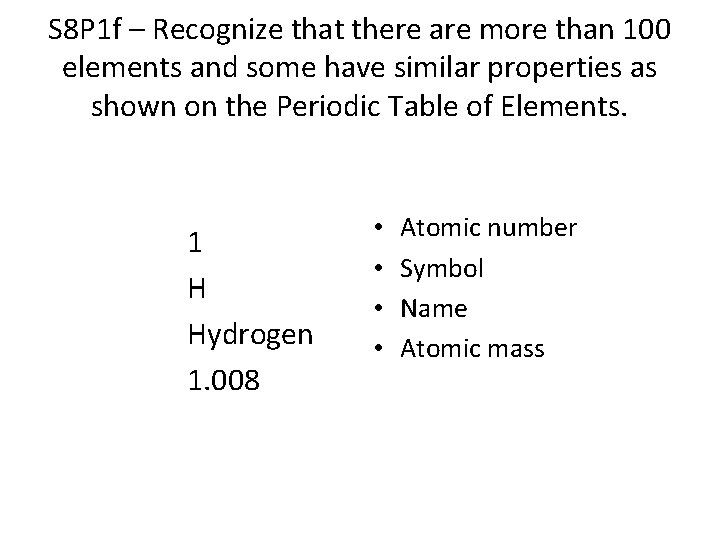
S 8 P 1 f – Recognize that there are more than 100 elements and some have similar properties as shown on the Periodic Table of Elements. 1 H Hydrogen 1. 008 • • Atomic number Symbol Name Atomic mass

S 8 P 1 f – Recognize that there are more than 100 elements and some have similar properties as shown on the Periodic Table of Elements. • What does the atomic number also tell? The number of protons and electrons an atom has. • List ways to determine if elements are similar. Use the group and period the element is in. • Groups – Columns on the Periodic Table that shows elements with the similar characteristics and atoms have the same number of valence electrons.

S 8 P 1 f – Recognize that there are more than 100 elements and some have similar properties as shown on the Periodic Table of Elements. • Periods – rows on the Periodic Table that show elements with varied properties. • Reactive Groups – Group 1 and the Halogens (Group 17) are the most reactive groups. • Noble Gases – Group 18 are noble gases because they are nonreactive due to not having any valence electrons.

S 8 P 1 f – Recognize that there are more than 100 elements and some have similar properties as shown on the Periodic Table of Elements. • Main Categories – 1. Metals – left side, mostly solids, most elements – 2. Nonmetals – right side, forms ions with the metals. – 3. Metalloids – staircase in the middle, metal and nonmetal characteristics

S 8 P 1 g – Identify and demonstrate the Law of Conservation of Matter • Law of Conservation of Matter – In a chemical reaction atoms are neither created or destroyed. • H 2 + O = H 2 O • Na + Cl = Na. Cl • Balance the following equation: Fe + S + 2 Fe. S

S 8 P 2: Students will be familiar in terms of the Law of Conservation of Energy.

S 8 P 2 a. Explain energy transformations in terms of the Law of Conservation of Energy. • Law of Conservation of Energy – Energy is neither created nor destroyed. • Example: A soccer ball is kicked. Energy transfers to the ball in the form of kinetic energy. The ball’s kinetic energy decreases as sound or heat from friction.

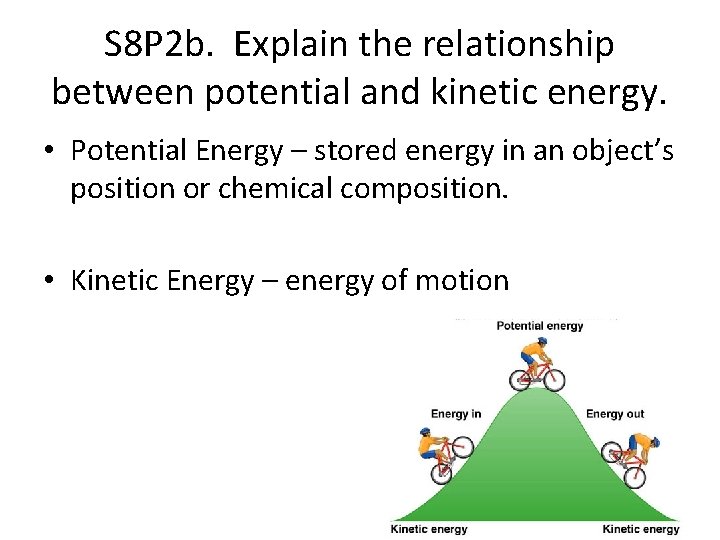
S 8 P 2 b. Explain the relationship between potential and kinetic energy. • Potential Energy – stored energy in an object’s position or chemical composition. • Kinetic Energy – energy of motion

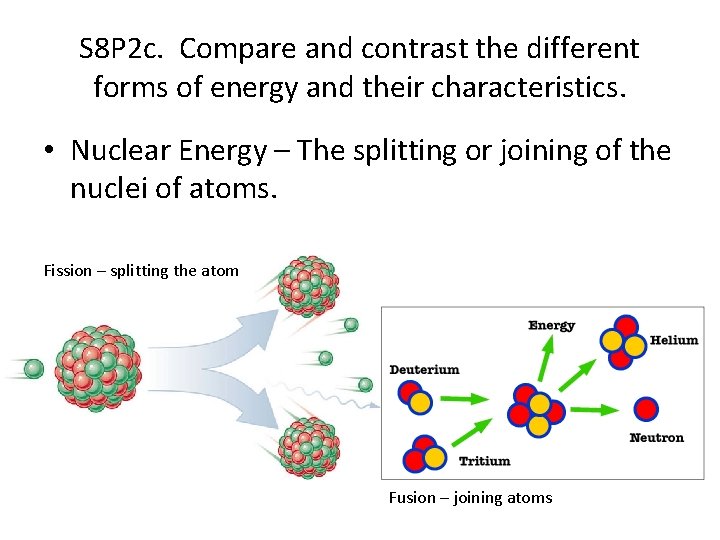
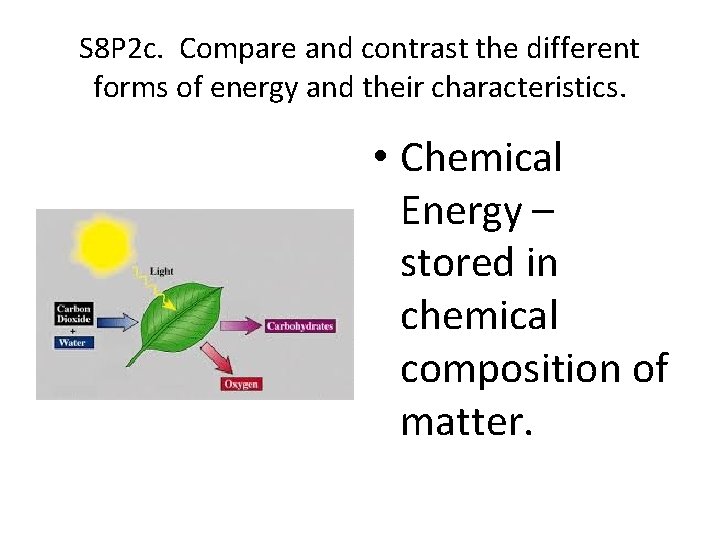
S 8 P 2 c. Compare and contrast the different forms of energy and their characteristics. • Nuclear Energy – The splitting or joining of the nuclei of atoms. Fission – splitting the atom Fusion – joining atoms

S 8 P 2 c. Compare and contrast the different forms of energy and their characteristics. • Thermal Energy – the total energy from movement of particles in matter.

S 8 P 2 c. Compare and contrast the different forms of energy and their characteristics. • Light Energy – Light from the sun that travels to earth by electromagnetic waves.

S 8 P 2 c. Compare and contrast the different forms of energy and their characteristics. • Electrical Energy – energy produced by movement of electrons.

S 8 P 2 c. Compare and contrast the different forms of energy and their characteristics. • Mechanical Energy – the energy in a moving object.

S 8 P 2 c. Compare and contrast the different forms of energy and their characteristics. • Sound Energy – energy from the vibration of particles in solids, liquids, and gases.

S 8 P 2 c. Compare and contrast the different forms of energy and their characteristics. • Chemical Energy – stored in chemical composition of matter.

S 8 P 2 d. Describe how heat can be transferred through matter by the collision of atoms (conduction), or through space (radiation). In liquid or gas, currents will facilitate the transfer of heat (convection). Conduction: Heat transfer by direct contact. ex. touching a hot stove Radiation: Heat transfer by electromagnetic waves from the sun. Ex. Heat from a campfire Convection: Heat rising. Ex. Heat felt from the hot pavement, heat over the stove

S 8 P 3 – Students will investigate relationships between force, mass, and the motion of objects.

S 8 P 3 a: Determine the relationship between velocity and acceleration • Acceleration: change in velocity as it changes over time. • Velocity: Speed in a specific direction. • S=d/t • Ex. A car speeds up, then slows down and stops at a red light. • Ex. Mousetrap calculations

S 8 P 3 b: Demonstrate the effect of balanced and unbalanced forces on an object in terms of gravity, inertia, and friction. • Unbalanced Forces – Changes the motion of an object. • Balanced Forces – No change in the objects motion.

S 8 P 3 b: Demonstrate the effect of balanced and unbalanced forces on an object in terms of gravity, inertia, and friction. • Gravity – The force that objects exert on each other because of their masses. • Inertia – The resistance of an object to change its motion. Newton’s First Law. • Friction – Force that resists the motion between two surfaces in contact.

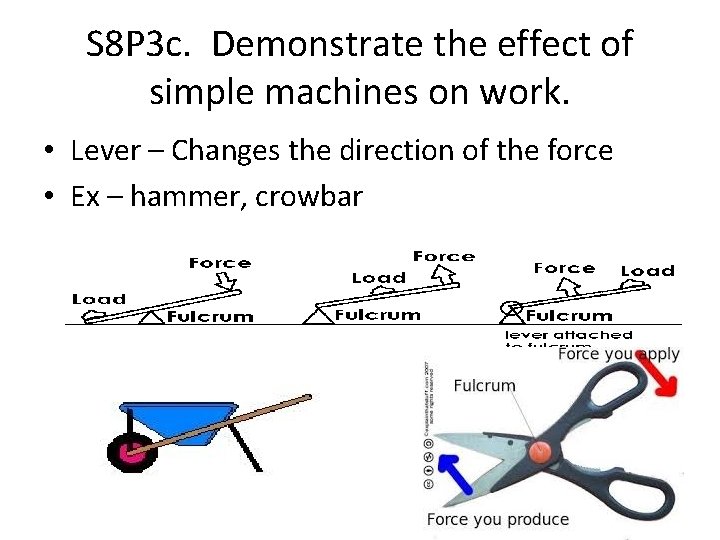
S 8 P 3 c. Demonstrate the effect of simple machines on work. • Lever – Changes the direction of the force • Ex – hammer, crowbar

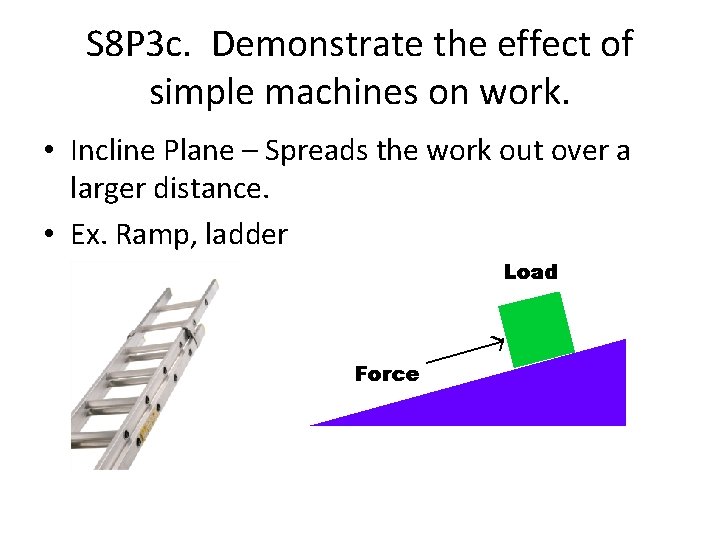
S 8 P 3 c. Demonstrate the effect of simple machines on work. • Incline Plane – Spreads the work out over a larger distance. • Ex. Ramp, ladder

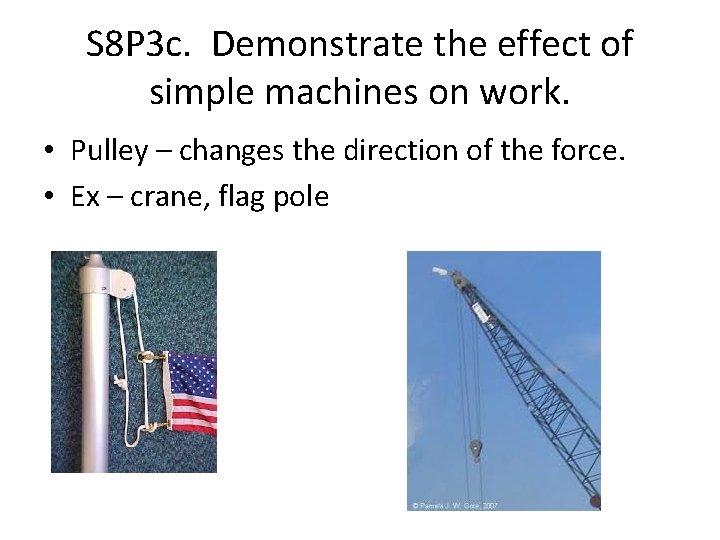
S 8 P 3 c. Demonstrate the effect of simple machines on work. • Pulley – changes the direction of the force. • Ex – crane, flag pole

S 8 P 3 c. Demonstrate the effect of simple machines on work. • Wedge – changes the direction by decreasing the surface area. • Ex. Knife blade, ax head

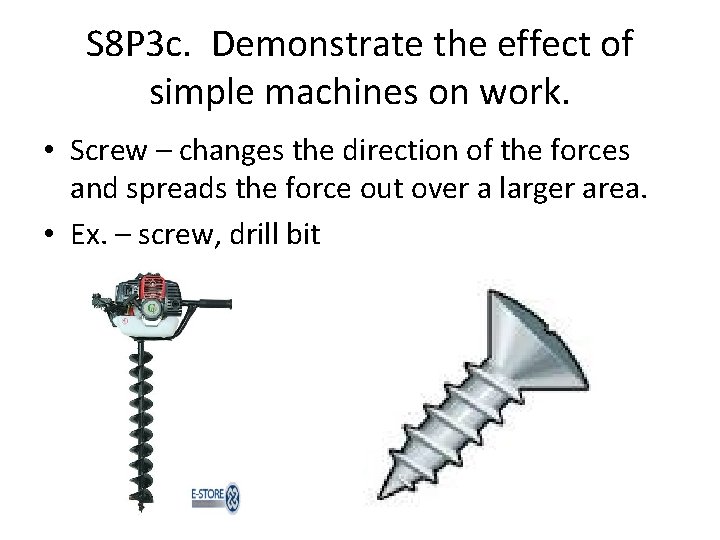
S 8 P 3 c. Demonstrate the effect of simple machines on work. • Screw – changes the direction of the forces and spreads the force out over a larger area. • Ex. – screw, drill bit

S 8 P 3 c. Demonstrate the effect of simple machines on work. • Wheel and axle – spreads workout over a larger area. • Ex. Turning plow, door knob

S 8 P 4: Students will explore the wave nature of sound and electromagnetic radiation.

S 8 P 4 a: Identify the characteristics of electromagnetic and mechanical waves. • Electromagnetic Waves that travel through space from the sun – travel through a vacuum. • Mechanical Waves that must move through a medium (matter) Examples: Visible light, microwaves, gamma rays, Example: sound

S 8 P 4 b: Describe how the behavior of light waves is manipulated causing reflection, refraction, diffraction, absorption. • Reflection: Waves bounce back. • Refraction: Waves bend. • Diffraction: Waves spread out around an object or opening. • Absorption: Waves disappear into a medium.

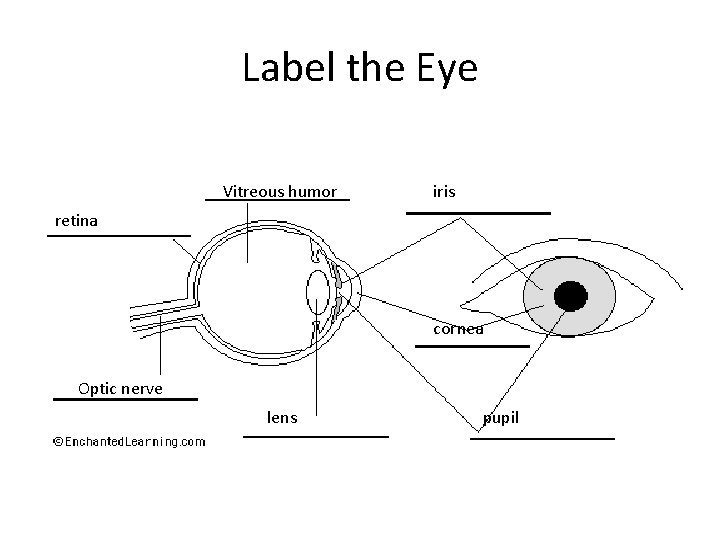
S 8 P 4 c. Explain how the human eye sees objects and colors in terms of wavelength. • How the eye works: An image is refracted through the cornea and lens, flips upside down, focuses on the retina which determines the color and image. The optic nerve carries the messages to the brain. • ROYGBIV: red, orange, yellow, green, blue, indigo, and violet. Red carries the least amount of energy with violet carrying the most amount of energy.

Label the Eye Vitreous humor iris retina cornea Optic nerve lens pupil

S 8 P 4 d. Describe how the behavior of waves is affected by mediums such as air, water, or solids. • Air – Transmits light and allows it to pass through. Sound will travel at a LOW rate. • Water – Refracts and/or reflects light. Sound will travel at a MEDIUM rate. • Solid – Reflects and/or absorbs light. Sound will travel at a HIGH rate.

S 8 P 4 e. Relate the properties of sound to everyday experiences. • Sound is used for communication and entertainment.

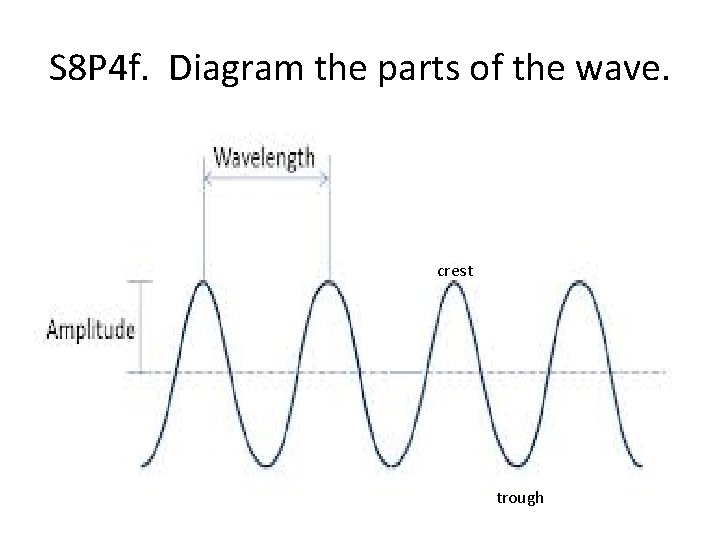
S 8 P 4 f. Diagram the parts of the wave. crest trough

S 8 P 4 f. Explain how the parts of a wave are affected by changes in amplitude and pitch. • An increase in amplitude will cause an increase in energy. • Pitch is determined by the frequency of a wave.

• Crest – If amplitude increases, the crest will Increase. If the pitch increases, the crest will Stay the Same. • Trough – If the amplitude increases, the trough will Increase. If the pitch increases, the trough will Stay the Same. • Wavelength – If the amplitude increases, the wavelength will Stay the same. If the pitch increases, the wavelength Decreases.

S 8 P 5: Students will recognize characteristics of gravity, electricity, and magnetism as major kinds of forces acting in nature.

S 8 P 5 a: Recognize the every object exerts gravitational force on every other object and that the force exerted depends on how much mass the objects have and how far apart they are. • Explain how this standard refers to planets and the effects of their gravity. Mass – greater mass = greater gravity Distance – as the distance increases, gravity decreases between 2 objects.

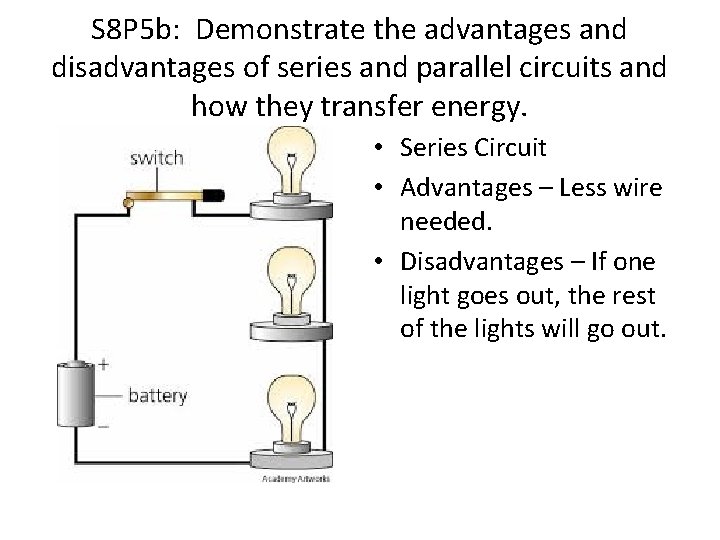
S 8 P 5 b: Demonstrate the advantages and disadvantages of series and parallel circuits and how they transfer energy. • Series Circuit • Advantages – Less wire needed. • Disadvantages – If one light goes out, the rest of the lights will go out.

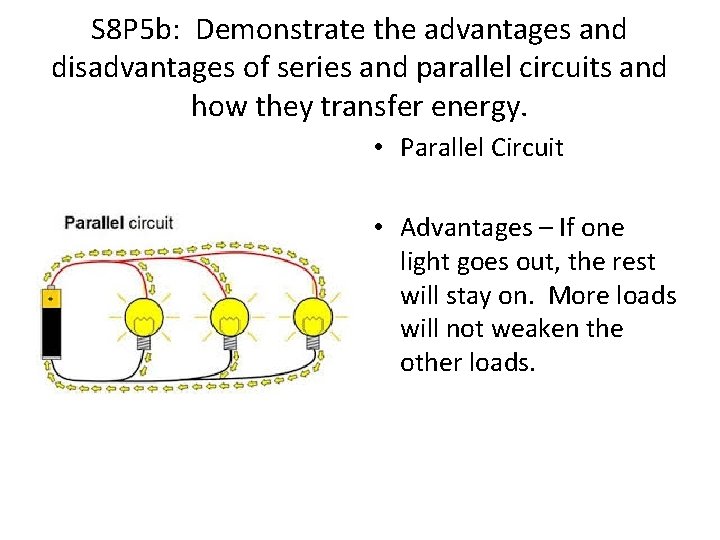
S 8 P 5 b: Demonstrate the advantages and disadvantages of series and parallel circuits and how they transfer energy. • Parallel Circuit • Advantages – If one light goes out, the rest will stay on. More loads will not weaken the other loads.

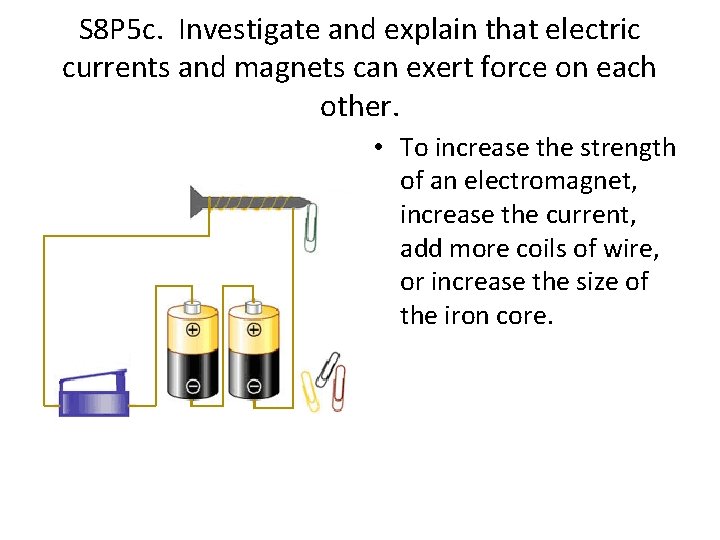
S 8 P 5 c. Investigate and explain that electric currents and magnets can exert force on each other. • To increase the strength of an electromagnet, increase the current, add more coils of wire, or increase the size of the iron core.

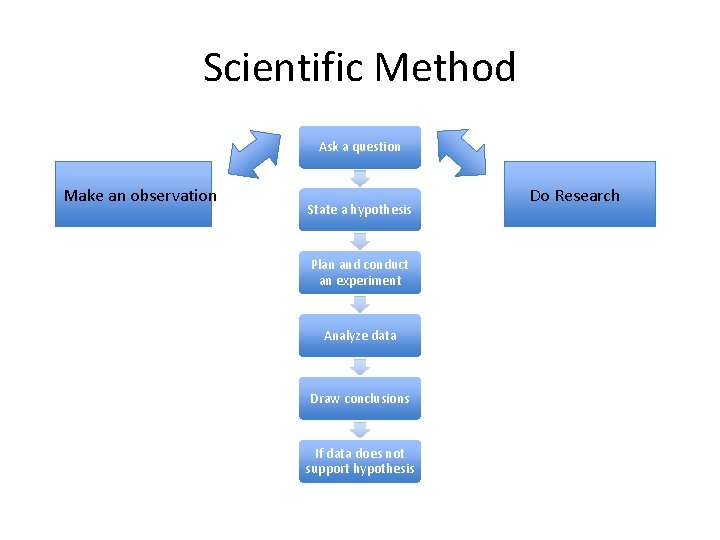
Scientific Method Ask a question Make an observation State a hypothesis Plan and conduct an experiment Analyze data Draw conclusions If data does not support hypothesis Do Research

Hypothesis • A possible answer to a scientific question that is based on logical reasoning and research.

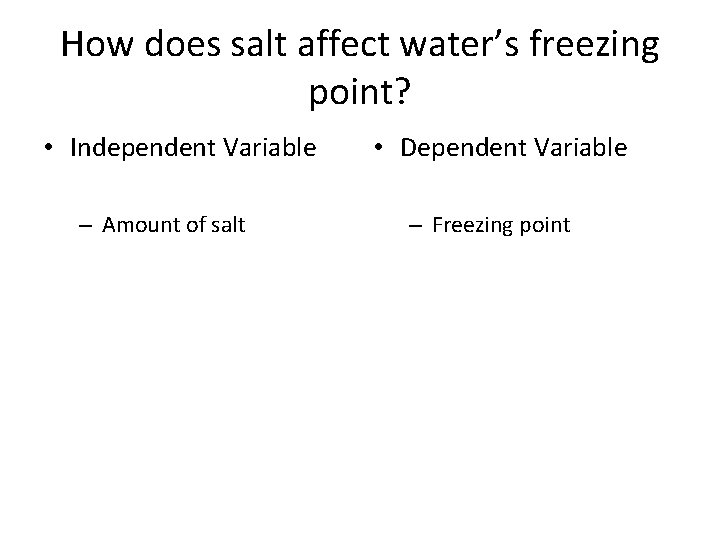
How does salt affect water’s freezing point? • Independent Variable – Amount of salt • Dependent Variable – Freezing point

Which fertilizer grows the largest apples? • Independent Variable – Type of fertilizer • Dependent Variable – Size of apple

What size parachute slows a free fall fastest? • Independent Variable • Dependent Variable – Area of parachute – Speed of free fall

Variables and Groups • Independent Variable – what is changed during an experiment. • Dependent Variable – what or who that responds to the change, what is being measured’ • Control Group – the normal, no change • Experimental Group – receives the independent variable, what or who is being studied.