5 Solids Liquids and Gases D Ideal Gases












- Slides: 43

5 Solids, Liquids and Gases “D Ideal Gases” 1 Forces and Motion 5 Solid Liquids Gases 2 Electricity 6 Magnetism 3 Waves 7 Radioactivity i. GCSE Edexcel 1 -9 – Mr Powell 4 Energy 8 Astrophysics

New Ideas to cover. . . Animated Science 2018

5 Solids, Liquids and Gases – “Part D” Animated Science 2018


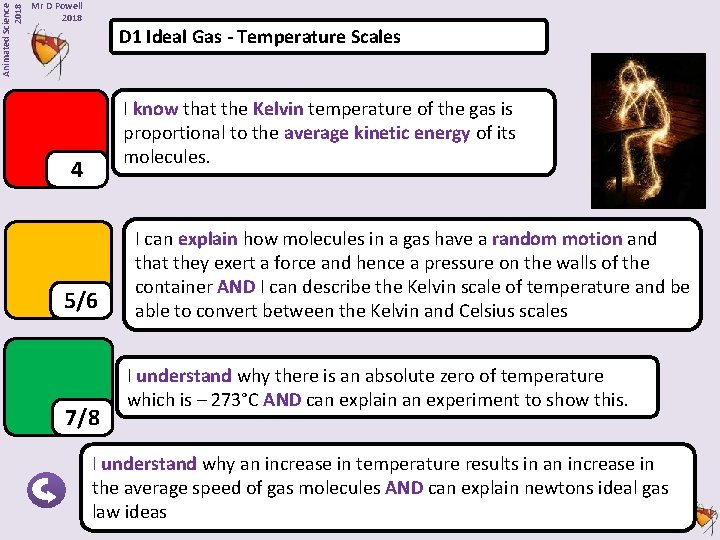
Animated Science 2018 Mr D Powell 2018 D 1 Ideal Gas - Temperature Scales I know that the Kelvin temperature of the gas is proportional to the average kinetic energy of its molecules. 4 5/6 7/8 I can explain how molecules in a gas have a random motion and that they exert a force and hence a pressure on the walls of the container AND I can describe the Kelvin scale of temperature and be able to convert between the Kelvin and Celsius scales I understand why there is an absolute zero of temperature which is – 273°C AND can explain an experiment to show this. I understand why an increase in temperature results in an increase in the average speed of gas molecules AND can explain newtons ideal gas law ideas Animated Science 2018

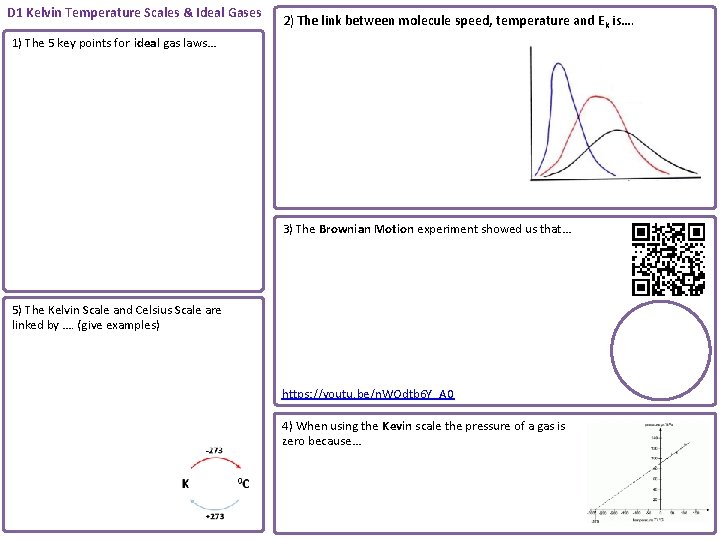
D 1 Kelvin Temperature Scales & Ideal Gases 2) The link between molecule speed, temperature and Ek is…. 1) The 5 key points for ideal gas laws… 3) The Brownian Motion experiment showed us that… 5) The Kelvin Scale and Celsius Scale are linked by …. (give examples) https: //youtu. be/n. WOdtb 6 Y_A 0 4) When using the Kevin scale the pressure of a gas is zero because…

The Random Motion of Gases & the Forces they exert on a container Firstly Charles and Boyle looked at gases via experimental methods then Robert Hooke and Isaac Newton’s thought about of the mechanics of each particle and its interaction with the walls of a chamber was combined to provide us with another take on how to explain how gases behave. They came up with some simple ideas… 1. Gas molecules have rapid and random motion. Newtons 3 laws… 1. Inertia - Objects carry on doing what they are already doing 2. Acceleration - F = ma 3. Equal and Opposites - force pairs of same type 2. When they collide with the walls of the container, they exert a force. 3. Pressure = Force/Area 4. There is a change of momentum Animated Science 2018

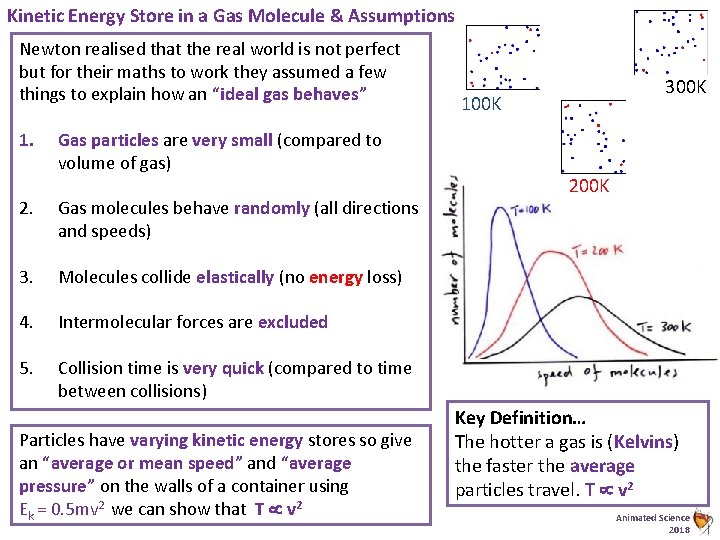
Kinetic Energy Store in a Gas Molecule & Assumptions Newton realised that the real world is not perfect but for their maths to work they assumed a few things to explain how an “ideal gas behaves” 1. Gas particles are very small (compared to volume of gas) 2. Gas molecules behave randomly (all directions and speeds) 3. Molecules collide elastically (no energy loss) 4. Intermolecular forces are excluded 5. Collision time is very quick (compared to time between collisions) Particles have varying kinetic energy stores so give an “average or mean speed” and “average pressure” on the walls of a container using Ek = 0. 5 mv 2 we can show that T v 2 300 K 100 K 200 K Key Definition… The hotter a gas is (Kelvins) the faster the average particles travel. T v 2 Animated Science 2018

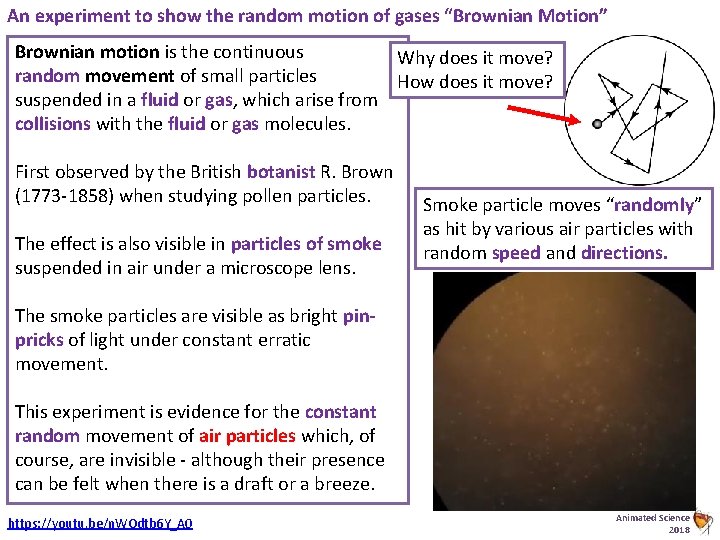
An experiment to show the random motion of gases “Brownian Motion” Brownian motion is the continuous Why does it move? random movement of small particles How does it move? suspended in a fluid or gas, which arise from collisions with the fluid or gas molecules. First observed by the British botanist R. Brown (1773 -1858) when studying pollen particles. The effect is also visible in particles of smoke suspended in air under a microscope lens. Smoke particle moves “randomly” as hit by various air particles with random speed and directions. The smoke particles are visible as bright pinpricks of light under constant erratic movement. This experiment is evidence for the constant random movement of air particles which, of course, are invisible - although their presence can be felt when there is a draft or a breeze. https: //youtu. be/n. WOdtb 6 Y_A 0 Animated Science 2018

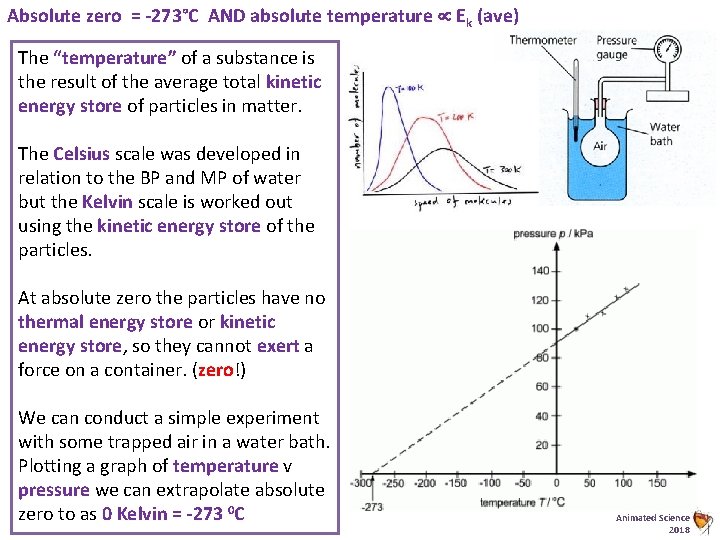
Absolute zero = -273°C AND absolute temperature Ek (ave) The “temperature” of a substance is the result of the average total kinetic energy store of particles in matter. The Celsius scale was developed in relation to the BP and MP of water but the Kelvin scale is worked out using the kinetic energy store of the particles. At absolute zero the particles have no thermal energy store or kinetic energy store, so they cannot exert a force on a container. (zero!) We can conduct a simple experiment with some trapped air in a water bath. Plotting a graph of temperature v pressure we can extrapolate absolute zero to as 0 Kelvin = -273 0 C Animated Science 2018

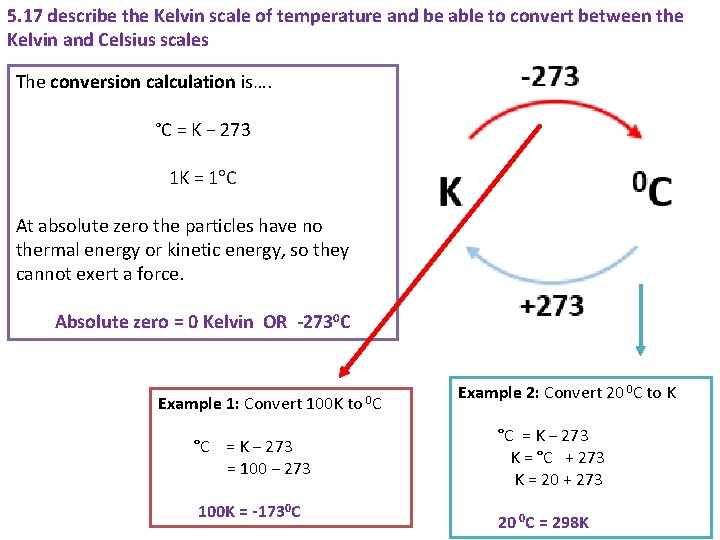
5. 17 describe the Kelvin scale of temperature and be able to convert between the Kelvin and Celsius scales The conversion calculation is…. °C = K − 273 1 K = 1 C At absolute zero the particles have no thermal energy or kinetic energy, so they cannot exert a force. Absolute zero = 0 Kelvin OR -2730 C Example 1: Convert 100 K to °C = K − 273 = 100 − 273 100 K = -1730 C 0 C Example 2: Convert 20 0 C to K °C = K − 273 K = °C + 273 K = 20 + 273 20 0 C = 298 K Animated Science 2018

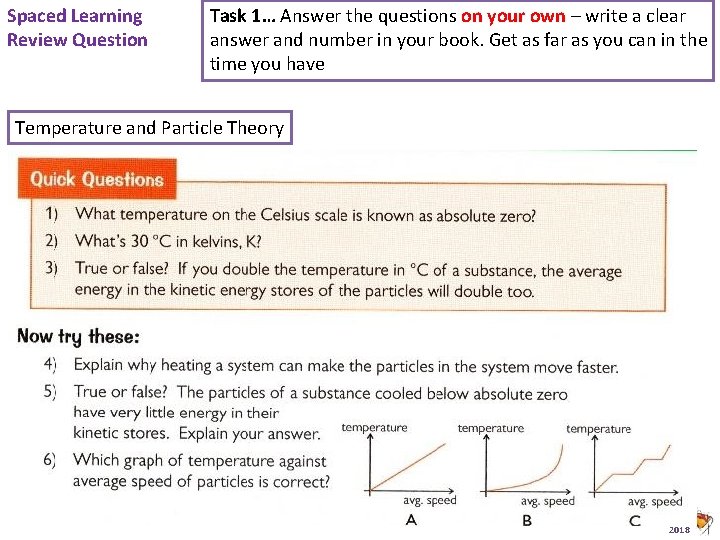
Spaced Learning Review Question Task 1… Answer the questions on your own – write a clear answer and number in your book. Get as far as you can in the time you have Temperature and Particle Theory Animated Science 2018

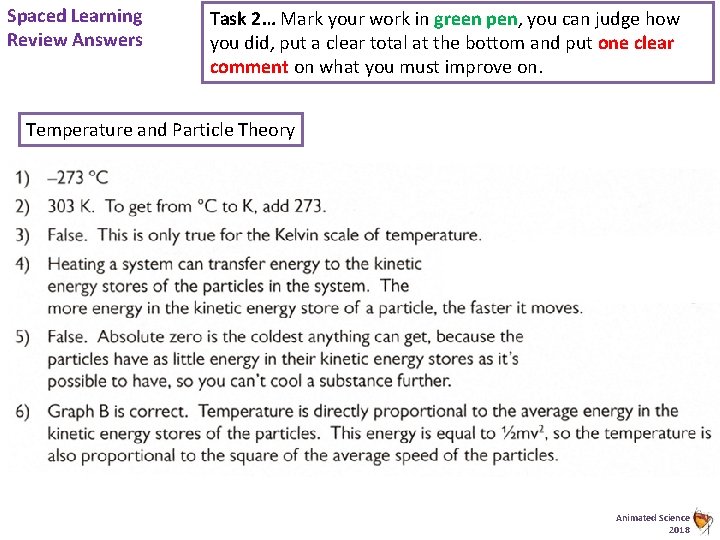
Spaced Learning Review Answers Task 2… Mark your work in green pen, you can judge how you did, put a clear total at the bottom and put one clear comment on what you must improve on. Temperature and Particle Theory Animated Science 2018

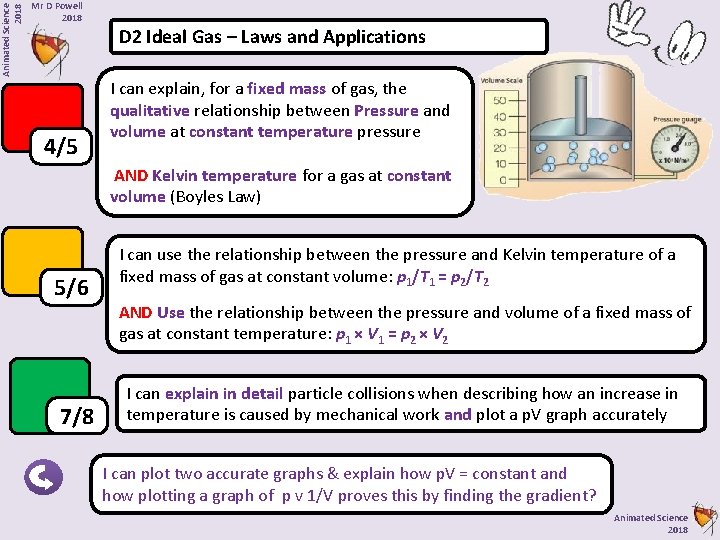
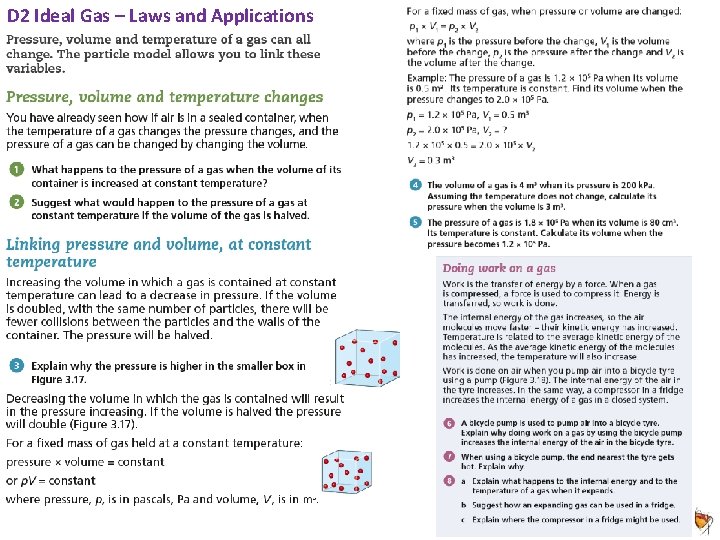
Animated Science 2018 Mr D Powell 2018 D 2 Ideal Gas – Laws and Applications 4/5 I can explain, for a fixed mass of gas, the qualitative relationship between Pressure and volume at constant temperature pressure AND Kelvin temperature for a gas at constant volume (Boyles Law) 5/6 7/8 I can use the relationship between the pressure and Kelvin temperature of a fixed mass of gas at constant volume: p 1/T 1 = p 2/T 2 AND Use the relationship between the pressure and volume of a fixed mass of gas at constant temperature: p 1 × V 1 = p 2 × V 2 I can explain in detail particle collisions when describing how an increase in temperature is caused by mechanical work and plot a p. V graph accurately I can plot two accurate graphs & explain how p. V = constant and how plotting a graph of p v 1/V proves this by finding the gradient? Animated Science 2018

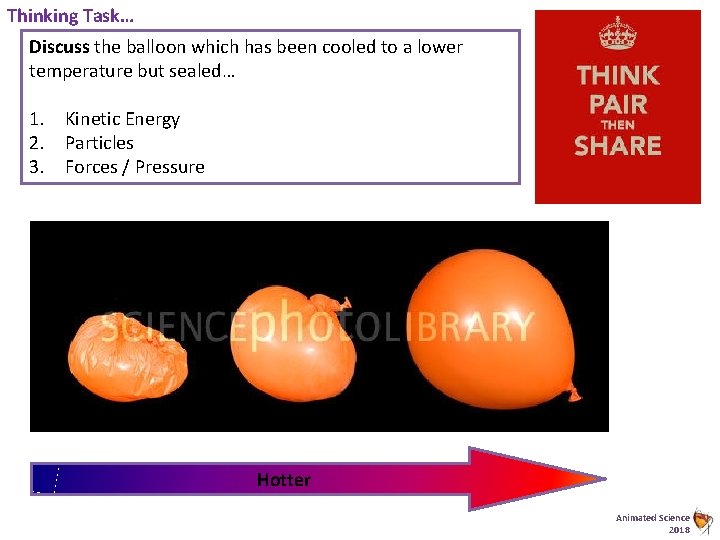
Thinking Task… Discuss the balloon which has been cooled to a lower temperature but sealed… 1. Kinetic Energy 2. Particles 3. Forces / Pressure Hotter Animated Science 2018

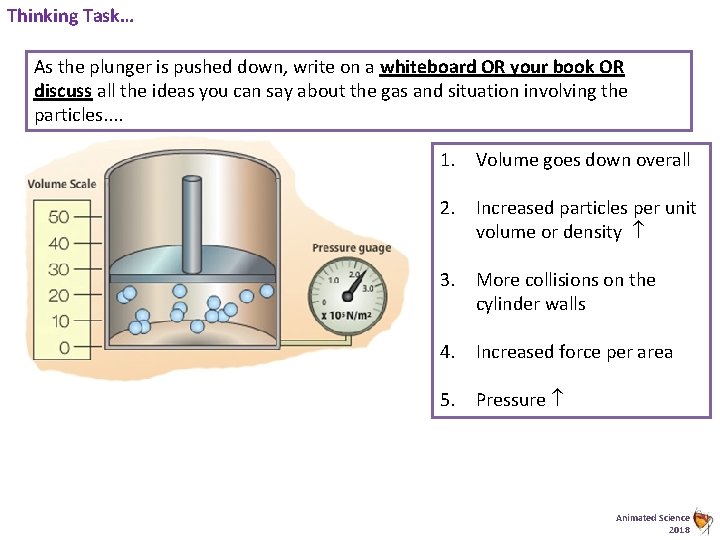
Thinking Task… As the plunger is pushed down, write on a whiteboard OR your book OR discuss all the ideas you can say about the gas and situation involving the particles. . 1. Volume goes down overall 2. Increased particles per unit volume or density 3. More collisions on the cylinder walls 4. Increased force per area 5. Pressure Animated Science 2018

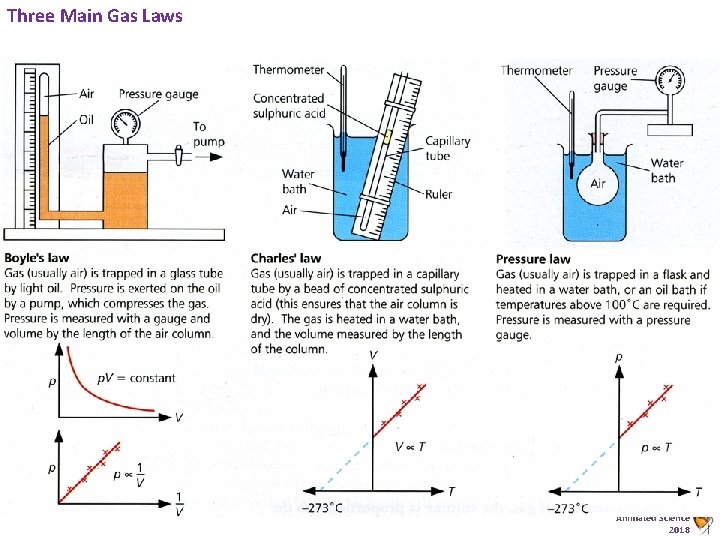
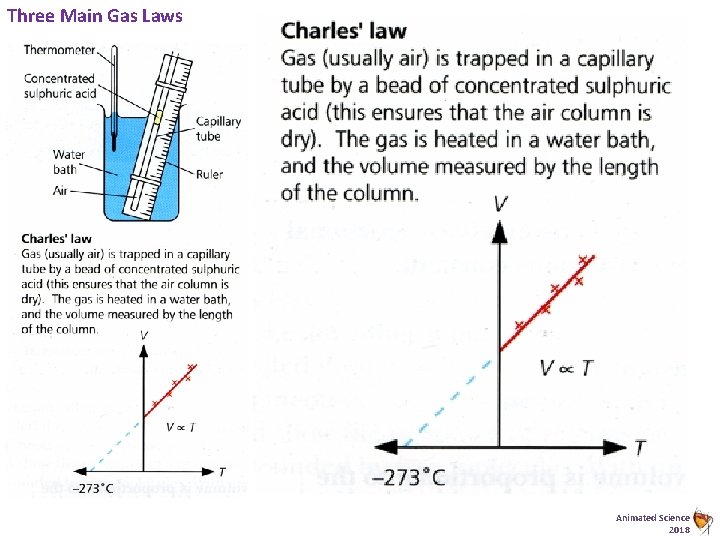
Three Main Gas Laws Animated Science 2018

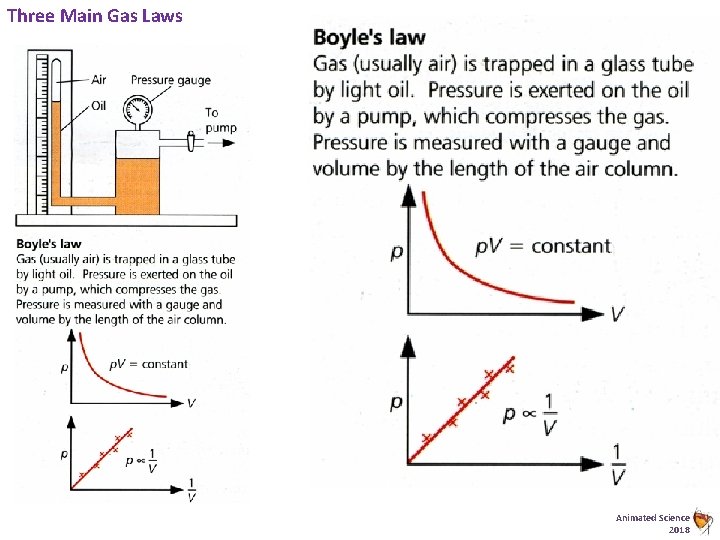
Three Main Gas Laws Animated Science 2018

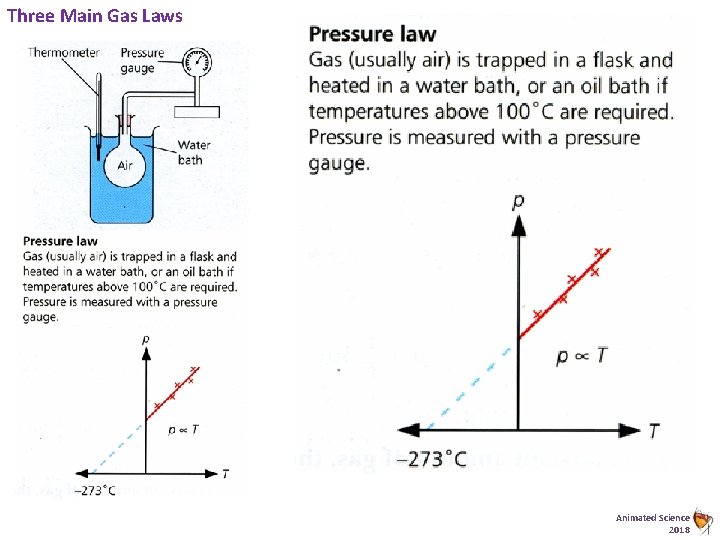
Three Main Gas Laws Animated Science 2018

Three Main Gas Laws Animated Science 2018

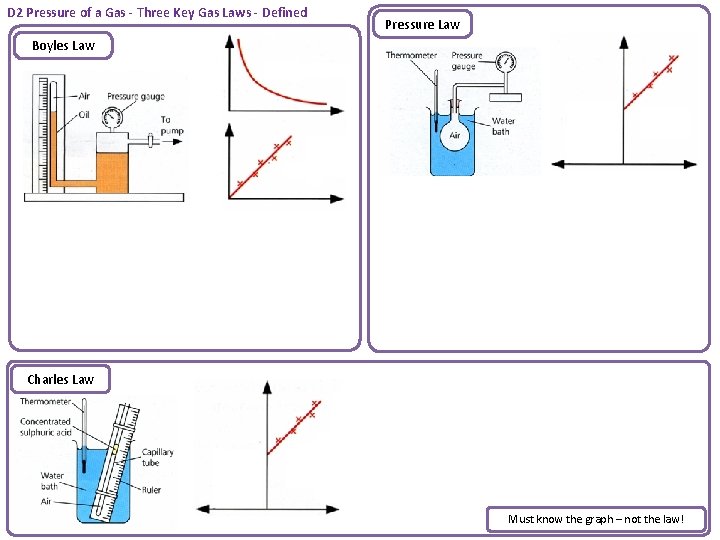
D 2 Pressure of a Gas - Three Key Gas Laws - Defined Pressure Law Boyles Law Charles Law Must know the graph – not the law!

Boyles Law – Challenge Practical Mass / kg Volume Syringe /ml 1 2 Mean 0. 2 4. 0 4 0. 4 4. 5 0. 6 5. 0 4. 9 5. 0 0. 8 5. 5 1. 0 6. 25 6. 3 Diameter Seal = 15. 25, 15. 23, 15. 25 mm Area of Seal = 1. 825 x 10 -4 m 2 Animated Science 2016

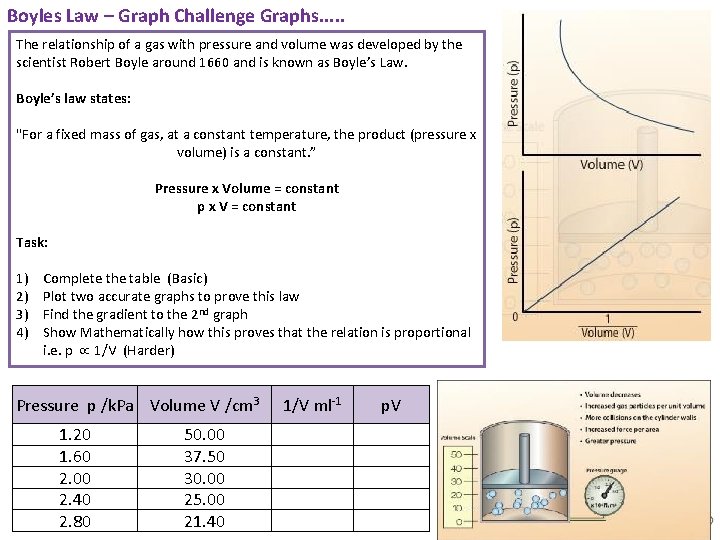
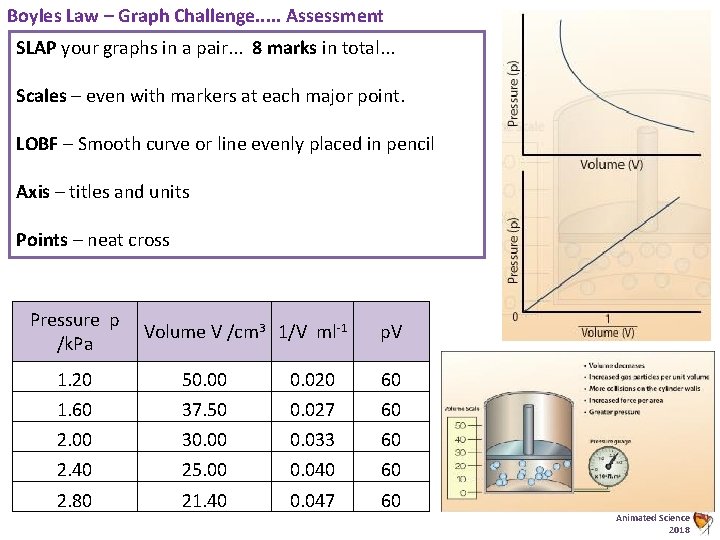
Boyles Law – Graph Challenge Graphs. . . The relationship of a gas with pressure and volume was developed by the scientist Robert Boyle around 1660 and is known as Boyle’s Law. Boyle’s law states: "For a fixed mass of gas, at a constant temperature, the product (pressure x volume) is a constant. ” Pressure x Volume = constant p x V = constant Task: 1) 2) 3) 4) Complete the table (Basic) Plot two accurate graphs to prove this law Find the gradient to the 2 nd graph Show Mathematically how this proves that the relation is proportional i. e. p 1/V (Harder) Pressure p /k. Pa Volume V /cm 3 1. 20 1. 60 2. 00 2. 40 2. 80 50. 00 37. 50 30. 00 25. 00 21. 40 1/V ml-1 p. V Animated Science 2018

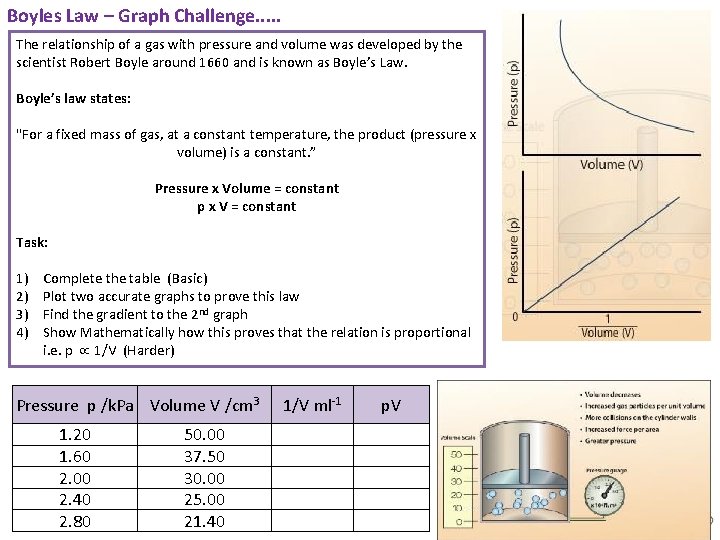
Boyles Law – Graph Challenge. . . The relationship of a gas with pressure and volume was developed by the scientist Robert Boyle around 1660 and is known as Boyle’s Law. Boyle’s law states: "For a fixed mass of gas, at a constant temperature, the product (pressure x volume) is a constant. ” Pressure x Volume = constant p x V = constant Task: 1) 2) 3) 4) Complete the table (Basic) Plot two accurate graphs to prove this law Find the gradient to the 2 nd graph Show Mathematically how this proves that the relation is proportional i. e. p 1/V (Harder) Pressure p /k. Pa Volume V /cm 3 1. 20 1. 60 2. 00 2. 40 2. 80 50. 00 37. 50 30. 00 25. 00 21. 40 1/V ml-1 p. V Animated Science 2018

Boyles Law – Graph Challenge. . . Assessment SLAP your graphs in a pair. . . 8 marks in total. . . Scales – even with markers at each major point. LOBF – Smooth curve or line evenly placed in pencil Axis – titles and units Points – neat cross Pressure p /k. Pa Volume V /cm 3 1/V ml-1 p. V 1. 20 50. 00 0. 020 60 1. 60 37. 50 0. 027 60 2. 00 30. 00 0. 033 60 2. 40 25. 00 0. 040 60 2. 80 21. 40 0. 047 60 Animated Science 2018

Boyles Law – Graph Challenge. . . Assessment Pressure p /k. Pa Volume V /cm 3 1/V ml-1 p. V 1. 20 50. 00 0. 020 60 1. 60 37. 50 0. 027 60 2. 00 30. 00 0. 033 60 2. 40 25. 00 0. 040 60 2. 80 21. 40 0. 047 60 Pressure p v 1/V Graph 3 2, 5 2 1, 5 1 0, 5 0 0 0, 005 0, 015 0, 025 0, 035 0, 045 0, 05 Animated Science 2018

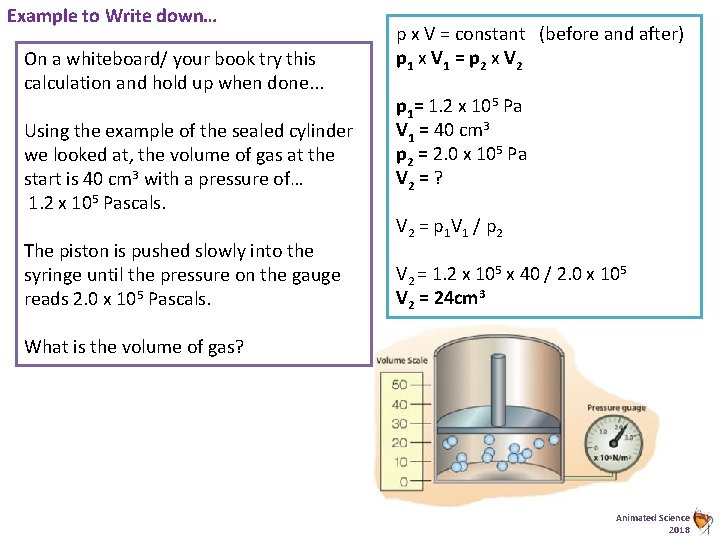
Example to Write down… On a whiteboard/ your book try this calculation and hold up when done. . . Using the example of the sealed cylinder we looked at, the volume of gas at the start is 40 cm 3 with a pressure of… 1. 2 x 105 Pascals. The piston is pushed slowly into the syringe until the pressure on the gauge reads 2. 0 x 105 Pascals. p x V = constant (before and after) p 1 x V 1 = p 2 x V 2 p 1= 1. 2 x 105 Pa V 1 = 40 cm 3 p 2 = 2. 0 x 105 Pa V 2 = ? V 2 = p 1 V 1 / p 2 V 2 = 1. 2 x 105 x 40 / 2. 0 x 105 V 2 = 24 cm 3 What is the volume of gas? Animated Science 2018

Pressure Law Example… Temp / °C 0 25 50 75 100 Pressure p /1 x 105 Pa 1. 0 1. 1 1. 2 1. 3 1. 4 Animated Science 2016

Pressure Law Example…. Temp / °C 1, 4 0 25 50 75 100 1, 35 Pressure/ 1*105 Pa 1, 3 1, 25 Pressure p /1 x 105 Pa 1 1. 2 1. 3 1. 4 1, 2 1, 15 1, 1 1, 05 1 0, 95 0, 9 0 50 Temp / °C 100 Animated Science 2016

Pressure Law Trent Real Data Temperature °C 2 15 25 37 43 Pressure law can also be used to find absolute zero. Think about the issues with the extrapolated data? Pressure p /k. Pa 90 95 100 105 108 Pressure Law - Linear Response 110 100 80 Pressure / k. Pa 105 100 95 60 40 20 90 0 -300 85 0 20 Temperature/°C 40 Temperature/°C Animated Science 2018

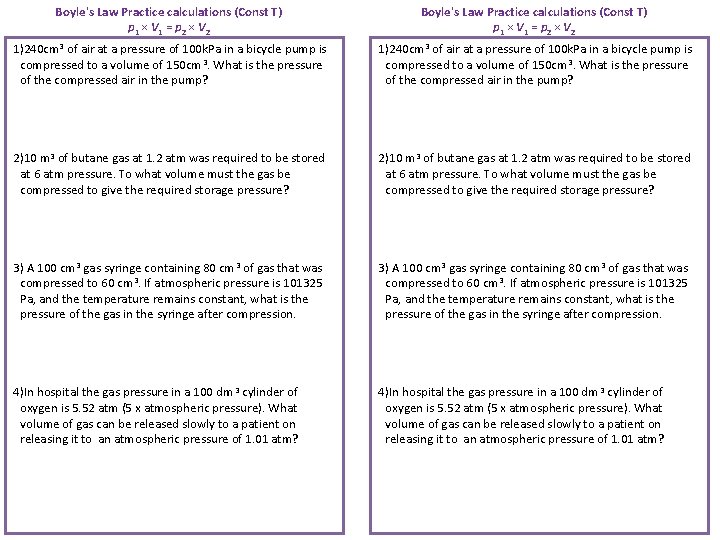
Boyle's Law Practice calculations (Const T) p 1 × V 1 = p 2 × V 2 1)240 cm 3 of air at a pressure of 100 k. Pa in a bicycle pump is compressed to a volume of 150 cm 3. What is the pressure of the compressed air in the pump? 2)10 m 3 of butane gas at 1. 2 atm was required to be stored at 6 atm pressure. To what volume must the gas be compressed to give the required storage pressure? 3) A 100 cm 3 gas syringe containing 80 cm 3 of gas that was compressed to 60 cm 3. If atmospheric pressure is 101325 Pa, and the temperature remains constant, what is the pressure of the gas in the syringe after compression. 4)In hospital the gas pressure in a 100 dm 3 cylinder of oxygen is 5. 52 atm (5 x atmospheric pressure). What volume of gas can be released slowly to a patient on releasing it to an atmospheric pressure of 1. 01 atm?

Boyle's Law Practice calculations (constant temperature assumed) p 1 × V 1 = p 2 × V 2 1) 240 cm 3 of air at a pressure of 100 k. Pa in a bicycle pump is compressed to a volume of 150 cm 3. What is the pressure of the compressed air in the pump? p 1 x V 1 = p 2 x V 2 ……………. rearranging to scale up for the new higher pressure p 2 = p 1 x V 1/V 2 = (100 x 240) / 150 = 160 k. Pa 2) 10 m 3 of butane gas at 1. 2 atm was required to be stored at 6 atm pressure. To what volume must the gas be compressed to give the required storage pressure? p 1 x V 1 = p 2 x V 2 ……… rearranging to scale down for the new lower volume V 2 = p 1 x V 1/p 2 = (1. 2 x 10) / 6 = 2. 0 m 3 Animated Science 2016

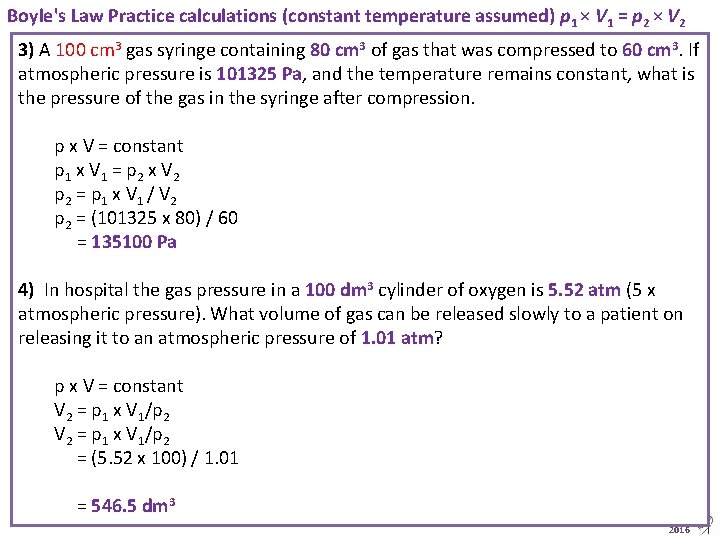
Boyle's Law Practice calculations (constant temperature assumed) p 1 × V 1 = p 2 × V 2 3) A 100 cm 3 gas syringe containing 80 cm 3 of gas that was compressed to 60 cm 3. If atmospheric pressure is 101325 Pa, and the temperature remains constant, what is the pressure of the gas in the syringe after compression. p x V = constant p 1 x V 1 = p 2 x V 2 p 2 = p 1 x V 1 / V 2 p 2 = (101325 x 80) / 60 = 135100 Pa 4) In hospital the gas pressure in a 100 dm 3 cylinder of oxygen is 5. 52 atm (5 x atmospheric pressure). What volume of gas can be released slowly to a patient on releasing it to an atmospheric pressure of 1. 01 atm? p x V = constant V 2 = p 1 x V 1/p 2 = (5. 52 x 100) / 1. 01 = 546. 5 dm 3 Animated Science 2016

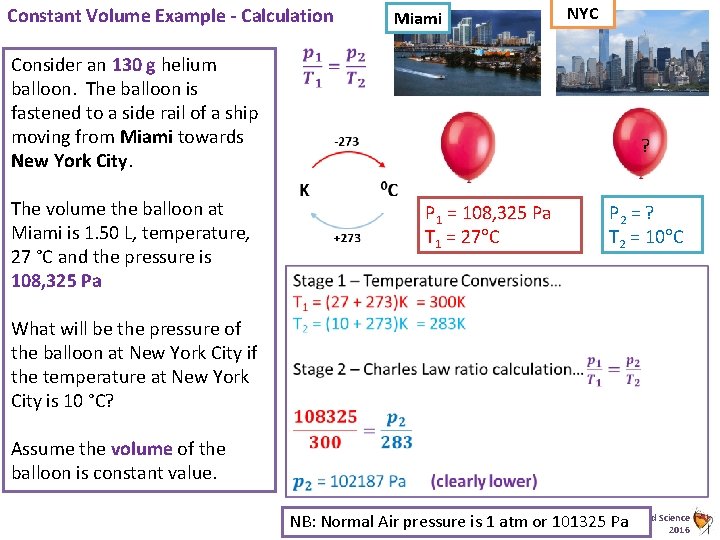
Constant Volume Example - Calculation Miami Consider an 130 g helium balloon. The balloon is fastened to a side rail of a ship moving from Miami towards New York City. The volume the balloon at Miami is 1. 50 L, temperature, 27 °C and the pressure is 108, 325 Pa NYC ? P 1 = 108, 325 Pa T 1 = 27 C P 2 = ? T 2 = 10 C What will be the pressure of the balloon at New York City if the temperature at New York City is 10 °C? Assume the volume of the balloon is constant value. Animated Science NB: Normal Air pressure is 1 atm or 101325 Pa 2016

Spaced Learning Review Question Task 1… Answer the questions on your own – write a clear answer and number in your book. Get as far as you can in the time you have Particle Theory & Gas Pressure Animated Science 2018

Spaced Learning Review Answers Task 2… Mark your work in green pen, you can judge how you did, put a clear total at the bottom and put one clear comment on what you must improve on. Particle Theory & Gas Pressure Animated Science 2018

D 2 Ideal Gas – Laws and Applications Animated Science 2018

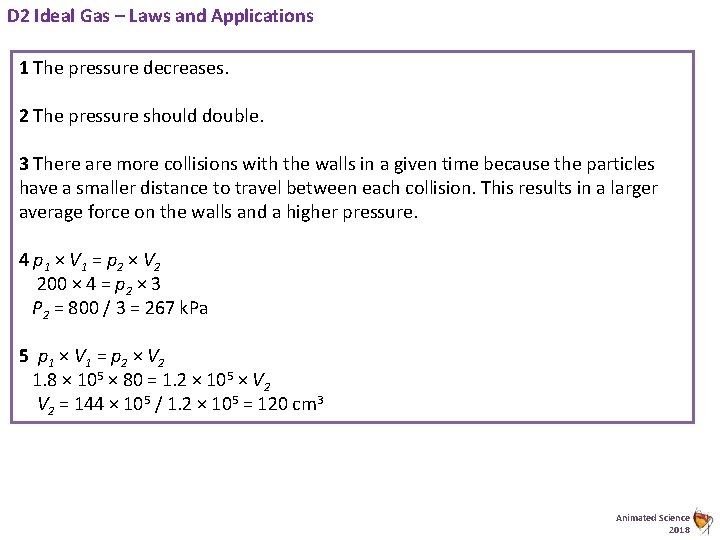
D 2 Ideal Gas – Laws and Applications 1 The pressure decreases. 2 The pressure should double. 3 There are more collisions with the walls in a given time because the particles have a smaller distance to travel between each collision. This results in a larger average force on the walls and a higher pressure. 4 p 1 × V 1 = p 2 × V 2 200 × 4 = p 2 × 3 P 2 = 800 / 3 = 267 k. Pa 5 p 1 × V 1 = p 2 × V 2 1. 8 × 105 × 80 = 1. 2 × 105 × V 2 = 144 × 105 / 1. 2 × 105 = 120 cm 3 Animated Science 2018

D 2 Ideal Gas – Laws and Applications 6 When you move the pump, the particles of gas colliding with the pump will end up moving faster. This increases the kinetic energy of the particles and so the internal energy increases. 7 The pump is doing work on the gas which heats up the gas. Since gas is not a very good conductor of heat, the region of the gas that gets hot remains near the pump. 8 a The internal energy of the gas must decrease since the gas is doing work as it expands. Therefore, the temperature must decrease. 8 b An expanding gas reduces the temperature. So you can make the gas expand inside a fridge to reduce the temperature of the fridge. 8 c The compressor increases the temperature of the gas. This is used on the outside of the fridge (at the back). As the cold, expanded gas moves inside the fridge, it gains internal energy as the warmer food heats it up a little. Then the gas passes to the outside of the fridge where it is compressed and heats up. The gas is now hotter than the surroundings and it loses internal energy (which has originally come from the food) to the surroundings by heating. Animated Science 2018

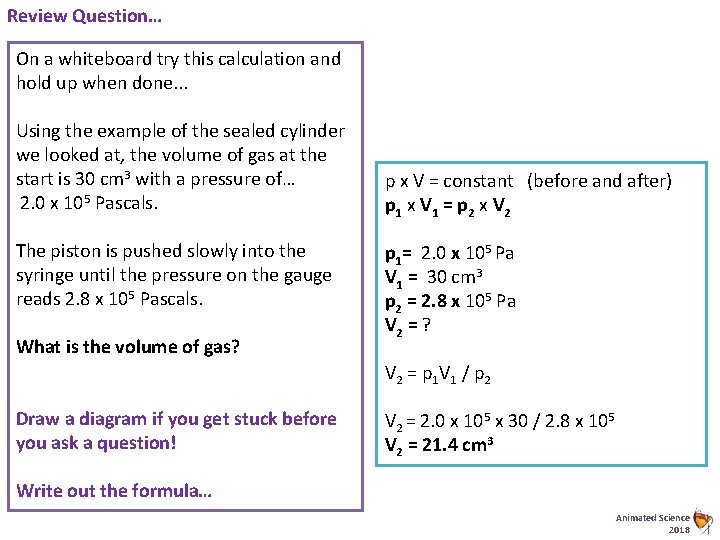
Review Question… On a whiteboard try this calculation and hold up when done. . . Using the example of the sealed cylinder we looked at, the volume of gas at the start is 30 cm 3 with a pressure of… 2. 0 x 105 Pascals. The piston is pushed slowly into the syringe until the pressure on the gauge reads 2. 8 x 105 Pascals. What is the volume of gas? p x V = constant (before and after) p 1 x V 1 = p 2 x V 2 p 1= 2. 0 x 105 Pa V 1 = 30 cm 3 p 2 = 2. 8 x 105 Pa V 2 = ? V 2 = p 1 V 1 / p 2 Draw a diagram if you get stuck before you ask a question! V 2 = 2. 0 x 105 x 30 / 2. 8 x 105 V 2 = 21. 4 cm 3 Write out the formula… Animated Science 2018

5 Points – Write a definition for the following words: • Melting • Freezing • Boiling • Evaporating • Condensing • Sublimating 2 Points – What is the conservation of mass? 2 Points – What is the difference in kinetic energy of water at 100°C and steam at 100°C ? 1 Point – What is the equation for density? 2 Points – Rearrange E=mcΘ for calculating mass. (T) 3 Points – Explain how you would calculate the density of an irregularly shaped object. 2 Points – What do these diagrams represent? 4 Points – Ethanol freezes at − 114 °C and boils at 78 °C. Draw a temperaturetime diagram for heating ethanol from − 120 °C to 100 °C. 2 Points – Write a definition for the specific heat capacity of a substance (T) 1 Point – What is the equation for specific heat capacity? 2 Points – Why do solids expand when heated? 2 Points – Draw particle diagrams for a solid, liquid and gas. 1 Point – What is internal energy? 2 Points – Why does a cork float but iron doesn’t? 2 Points – Explain the kinetic energy of a solid compared to a gas. Physics Chapter 5 – Solid Liquid Gas These questions are designed to help you revise this topic. The easier questions are worth less points. The Harder questions are worth more points. Write them out in full in your book and add up the points as you go. The least amount of points you need is dependent on your target grade: 4 – 24 points 5 – 28 points 6 – 32 points 7 – 38 points 8 – 45 points 3 Points – Why after evaporation is the remaining liquid cooler? 2 Points – A block of ice at 2 °C was heated. After 7304 J of energy were transferred to the ice, the ice had melted and reached a temperature of 5 °C. Calculate the mass of the ice. (T) 3 Points – • Which of the materials below requires the most energy to raise its temperature by 1 °C? (T) • Which substance can store the least energy? (T) 1 Point – Why do gases have a low density? 2 Points – In terms of particles, how goes a gas exert pressure? 1 Point – What is Θ ? (T) 1 Point – What happens to the internal energy of a liquid when heated? 3 Points – In terms of energy, why does the pressure of a gas increase as temperature increases? 2 Points – Why is there no change in temperature when ice melts? (T) 2 Points – Explain how the collapsing can experiment works. 2 Points – What happens to the molecules of a gas when heated? 2 Points – What is likely to happen when a gas looses kinetic energy? 1 Point – What is the latent heat of vaporisation? (T) 3 Points – Explain how to calculate the density of cube.

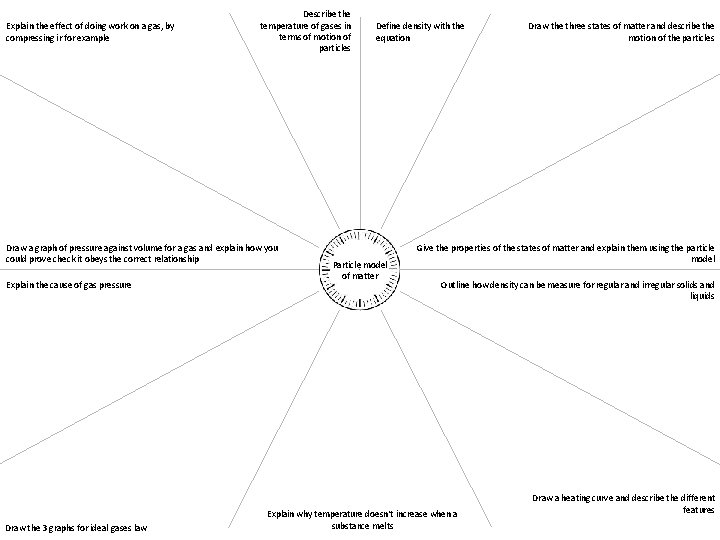
Explain the effect of doing work on a gas, by compressing ir for example Describe the temperature of gases in terms of motion of particles Draw a graph of pressure against volume for a gas and explain how you could prove check it obeys the correct relationship Explain the cause of gas pressure Draw the 3 graphs for ideal gases law Define density with the equation Particle model of matter Draw the three states of matter and describe the motion of the particles Give the properties of the states of matter and explain them using the particle model Outline how density can be measure for regular and irregular solids and liquids Explain why temperature doesn't increase when a substance melts Draw a heating curve and describe the different features

TOPIC 5 – Solid Liquid Gas Explain how a gas causes pressure on the walls of a container… Explain how to work out density in 5 simple steps… Fill in the diagram and label the arrows: Solid The specific latent heat of fusion for water is 334000 J/kg. An ice cube of mass 25 g melts at 0 o. C. How much energy is needed to melt the ice cube? (T) Gas Explain with a diagram the origin of gas pressure. Describe how the equipment below can be used to determine the density of the rock Key words Describe what is happening, in terms of particles, at the following points A-B D-E B-C E-F C-D Liquid
 Expansion of solids liquids and gases examples
Expansion of solids liquids and gases examples Solids liquids and gases section 2 properties of fluids
Solids liquids and gases section 2 properties of fluids States of matter venn diagram
States of matter venn diagram Solid
Solid Combined gas law def
Combined gas law def The science duo physical and chemical changes
The science duo physical and chemical changes Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Chapter 14 solids liquids and gases
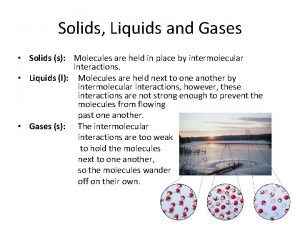
Chapter 14 solids liquids and gases Particle movement in solids liquids and gases
Particle movement in solids liquids and gases How does sound travel through solids liquids and gases
How does sound travel through solids liquids and gases Liquid information
Liquid information Motion of particles in solids, liquids and gases
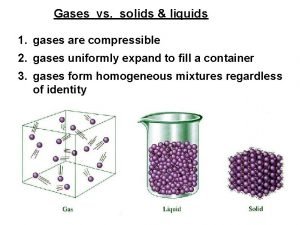
Motion of particles in solids, liquids and gases Why are gases eaiser to compress than solids or liquids?
Why are gases eaiser to compress than solids or liquids? Solid
Solid Adhesive force
Adhesive force Liquids and solids menu
Liquids and solids menu Liquids and solids
Liquids and solids Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids Constant rate filtration example
Constant rate filtration example Molecular theory of gases and liquids
Molecular theory of gases and liquids Ideal solution and non ideal solution
Ideal solution and non ideal solution Kinetic molecular model of gases
Kinetic molecular model of gases Characteristics of ideal gases
Characteristics of ideal gases Ideal gases characteristics
Ideal gases characteristics Compressibility of solid liquid and gas
Compressibility of solid liquid and gas First law of thermodynamics
First law of thermodynamics Mother sauces names
Mother sauces names A yolk and cream mixture that is used to thicken liquids *
A yolk and cream mixture that is used to thicken liquids * Classification of liquid dielectrics
Classification of liquid dielectrics Separates liquids of different colours
Separates liquids of different colours Parent rock of quartzite
Parent rock of quartzite 2 properties of a liquid
2 properties of a liquid What is a gas
What is a gas Ionic liquids ppt
Ionic liquids ppt Class b fires include
Class b fires include Regressive assimilation
Regressive assimilation The monophasic liquid
The monophasic liquid Main ingredients in baking
Main ingredients in baking Pull examples
Pull examples Compressible and incompressible fluids
Compressible and incompressible fluids Viscosity of liquids
Viscosity of liquids Miscible liquids
Miscible liquids Solid liquid and gas
Solid liquid and gas Split-tube thief
Split-tube thief