Viscosity conductance Part 10 3 4 2013 Introduction




![Intrinsic Viscosity ([η]): The ratio of a solution’s specific viscosity to the concentration Intrinsic Viscosity ([η]): The ratio of a solution’s specific viscosity to the concentration](https://slidetodoc.com/presentation_image/3a326d1486ac253754d25ec46df1095a/image-19.jpg)
















- Slides: 51

Viscosity & conductance Part 10 3 -4 -2013

Introduction Viscosity is a quantitative measure of a fluid’s resistance to flow. Dynamic (or Absolute) Viscosity: The dynamic viscosity(η) of a fluid is a measure of the resistance it offers to relative shearing motion ﺍﻟﺤﺮﻛﺔ ﺍﻟﻘﺼﻴﺔ. η= F/ [A×(u/h)] η= τ /(u/h) N-s/m² Kinematic Viscosity : It is defined as the ratio of absolute viscosity to the density of fluid. ν= η/ρ m²/s ; ρ= density of fluid

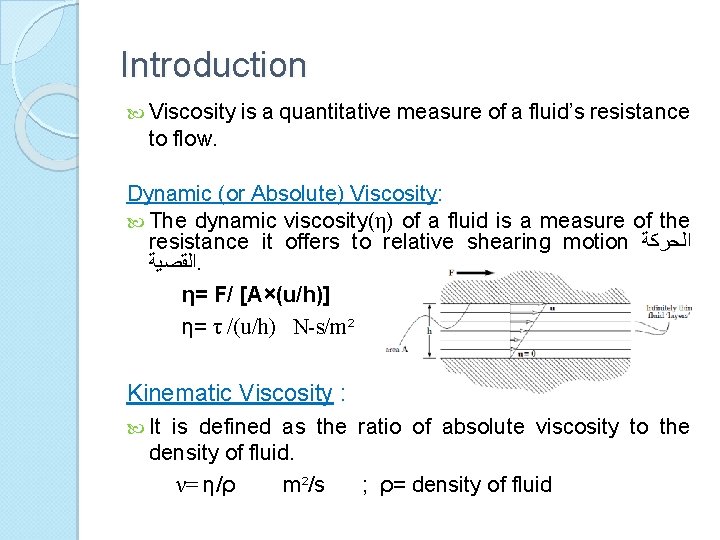
Viscosity Measurements Capillary Viscometers It gives the ‘kinematic viscosity’ of the fluid. It is based on Poiseuille’s law for steady viscous flow in a pipe.

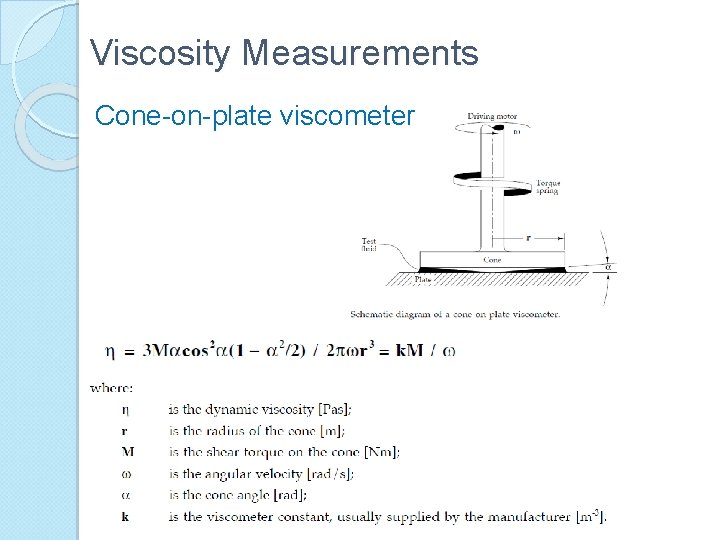
Viscosity Measurements Rotational Viscometers These viscometer give the value of the ‘dynamic viscosity’. It is based on the principle that the fluid whose viscosity is being measured is sheared between two surfaces. In these viscometers one of the surfaces is stationary and the other is rotated by an external drive and the fluid fills the space in between. The measurements are conducted by applying either a constant torque and measuring the changes in the speed of rotation or applying a constant speed and measuring the changes in the torque. There are two main types of these viscometers: rotating cylinder and cone-on-plate viscometers

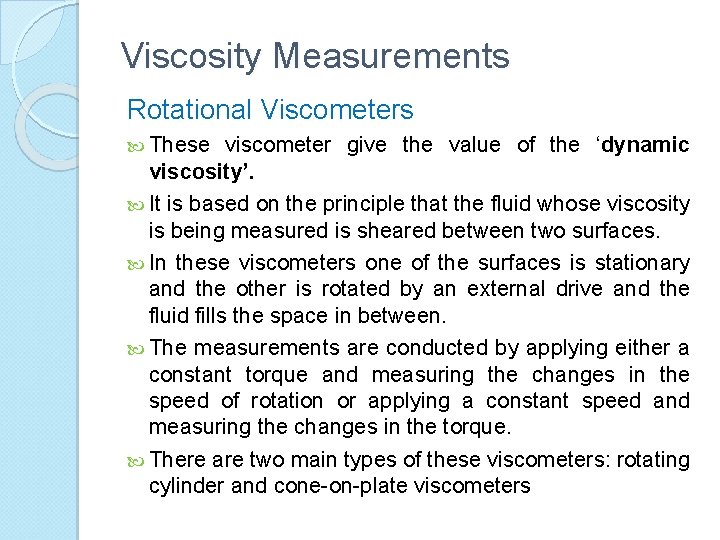
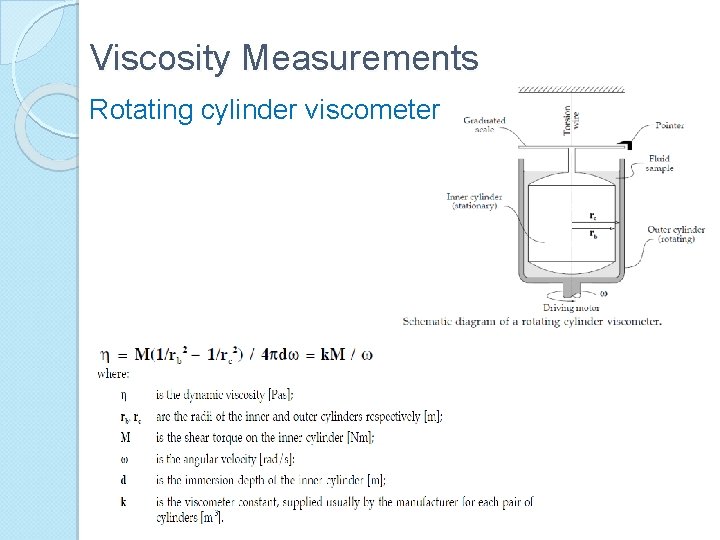
Viscosity Measurements Rotating cylinder viscometer

Viscosity Measurements Cone-on-plate viscometer

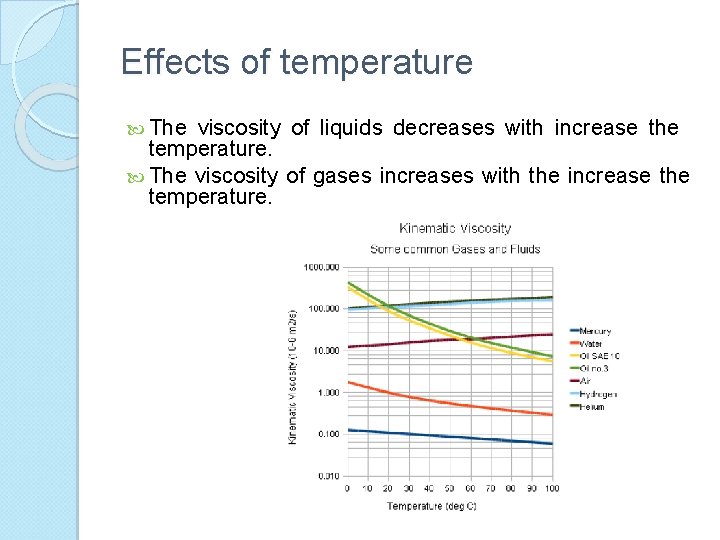
Effects of temperature The viscosity of liquids decreases with increase the temperature. The viscosity of gases increases with the increase the temperature.

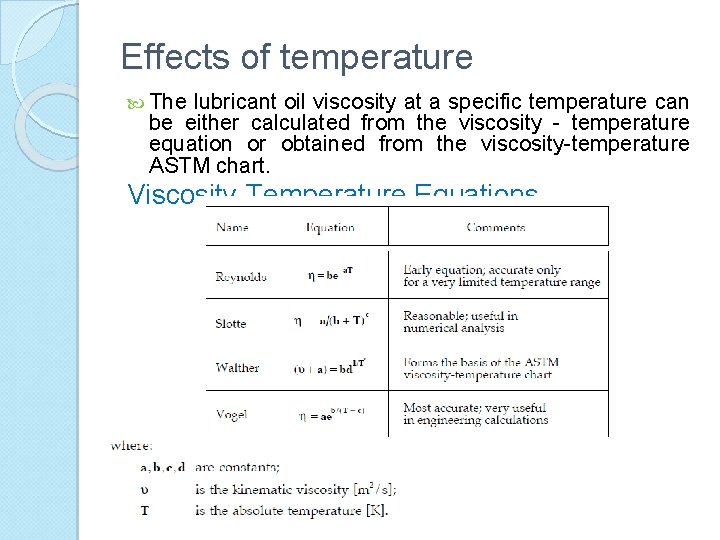
Effects of temperature The lubricant oil viscosity at a specific temperature can be either calculated from the viscosity - temperature equation or obtained from the viscosity-temperature ASTM chart. Viscosity-Temperature Equations

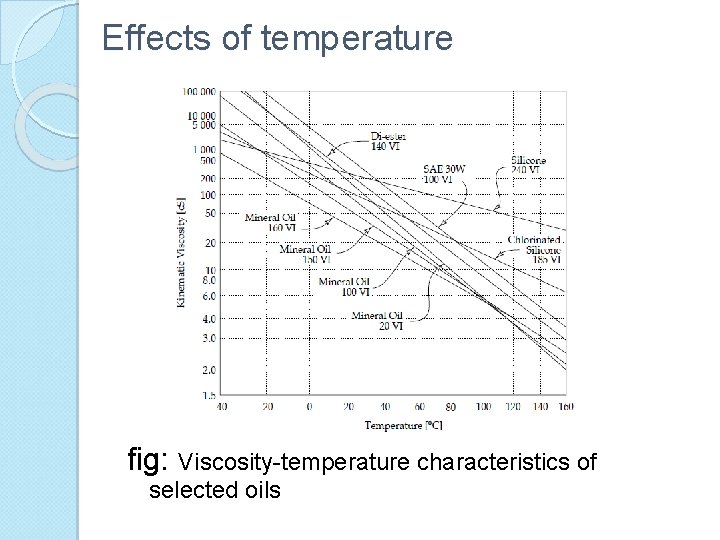
Effects of temperature fig: Viscosity-temperature characteristics of selected oils

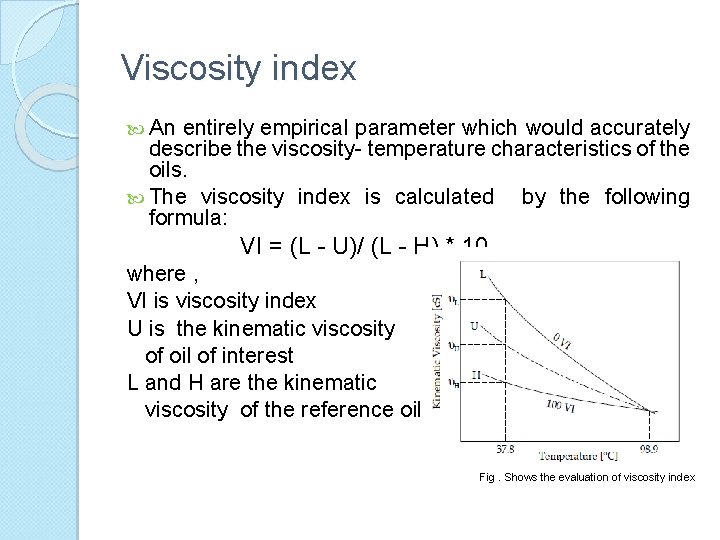
Viscosity index An entirely empirical parameter which would accurately describe the viscosity- temperature characteristics of the oils. The viscosity index is calculated by the following formula: VI = (L - U)/ (L - H) * 10 where , VI is viscosity index U is the kinematic viscosity of oil of interest L and H are the kinematic viscosity of the reference oils Fig. Shows the evaluation of viscosity index

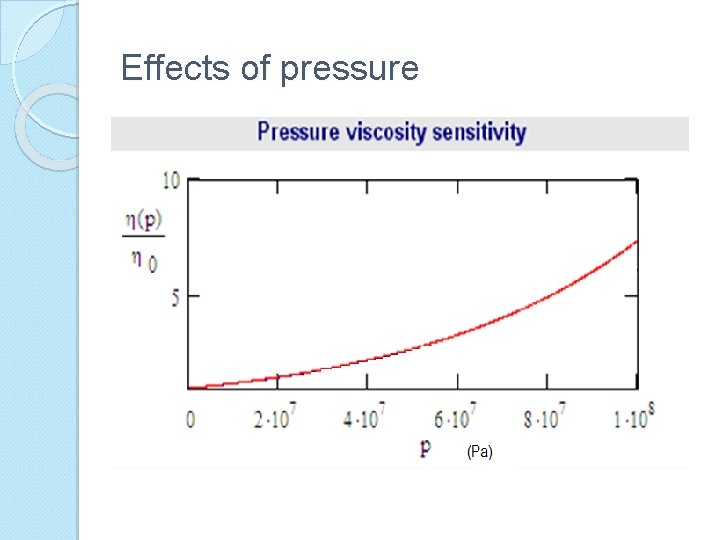
Effects of pressure Lubricants viscosity increases with pressure. For most lubricants this effect is considerably largest than the other effects when the pressure is significantly above atmospheric. The Barus equation :

Effects of pressure

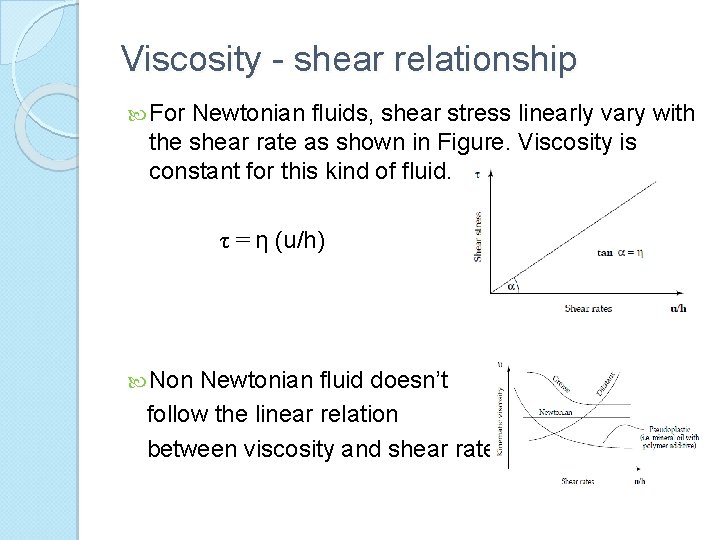
Viscosity - shear relationship For Newtonian fluids, shear stress linearly vary with the shear rate as shown in Figure. Viscosity is constant for this kind of fluid. τ = η (u/h) Non Newtonian fluid doesn’t follow the linear relation between viscosity and shear rate.

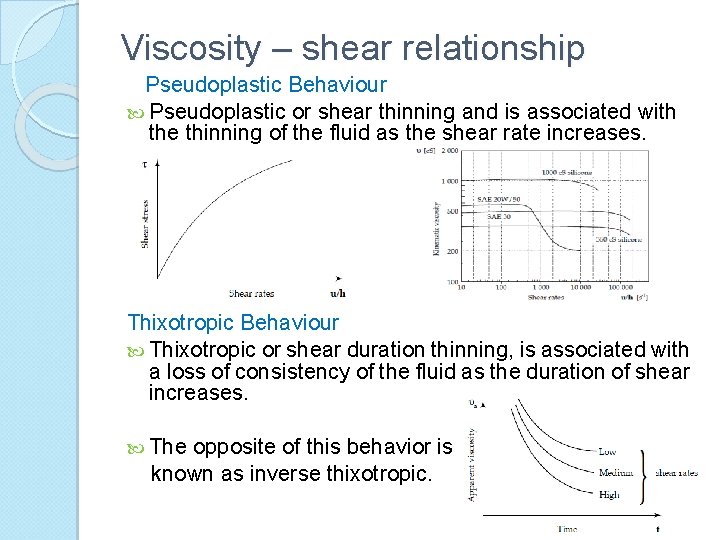
Viscosity – shear relationship Pseudoplastic Behaviour Pseudoplastic or shear thinning and is associated with the thinning of the fluid as the shear rate increases. Thixotropic Behaviour Thixotropic or shear duration thinning, is associated with a loss of consistency of the fluid as the duration of shear increases. The opposite of this behavior is known as inverse thixotropic.

Applications Selection of lubricants for various purpose. - we can choose an optimum range of viscosity for engine oil. - for high load and also for speed operation high viscous lubricants is required. In pumping operation - for high viscous fluid high power will require. - for low viscous fluid low power will require. In making of blend fuel - less viscous fuels easy to mix. In the operation of coating and printing.

Viscosity definations A measure of the resistance of flow due to internal friction when one layer of fluid is caused to move in relationship to another layer. The Poise represents absolute viscosity, the tangential force per unit area of either of two horizontal planes at unit distance apart, the space between being filled with the substance. A liquid with an absolute viscosity of one Poise requires a force of one dyne to maintain a velocity differential of one centimeter per second over a surface one centimeter square. When the ratio of shearing stress to the rate of shear is constant, as is the case with water and thin motor oils, the fluid is called a Newtonian fluid. In the case of non. Newtonian fluids, the ratio varies with the shearing stress, and viscosities of such fluids are called apparent viscosities. In the new SI system, it is proposed that values for the Poise be stated as Pascal seconds, the conversion factor being 1 Poise equal to 1 × 10 -1 Pa·s. A common measurement unit is the milli. Pascal second (m. Pa·s). Conversion factors are as follows: 1 centipoise (c. P) = 0. 01 poise (P) 1 Pa·s = 10 P 1 c. P = 0. 001 Pa·s = 1 m. Pa·s 1 Pa·s = 1000 c. P

Viscosities Absolute Viscosity: The tangential force per unit area of two parallel planes at unit distance apart when the space between them is filled with a fluid and one plane moves with unit velocity in its own plane relative to the other. Also known as coefficient of viscosity. Apparent Viscosity : The value obtained by applying the instrumental equations used in obtaining the viscosity of a Newtonian fluid to viscometer measurements of a non. Newtonian fluid. . Dilute Solution Viscosity: The viscosity of a dilute solution of a polymer, measured under prescribed conditions, is an indication of the molecular weight of the polymer and can be used to calculate the degree of polymerization

Kinematic Viscosity: The absolute viscosity of a fluid divided by the density of the fluid. Also known as the coefficient of kinematic viscosity. Measured in stokes (St) or centistokes (c. St). One centistoke = 0. 01 stokes. The metric unit is square metres per second (m 2/s). A common metric measurement unit in glass capillary viscometry is millimeters squared per second (mm 2/s). Conversion factors are as follows: 1 St = 1 x 10 -4 m 2/s 1 m 2/s = 10, 000 St 1 c. St = 1 x 10 -6 m 2/s = 1 mm 2/s 1 m 2/s = 1, 000 c. St Centistokes may be converted to centipoise (c. P) by multiplying by the density of the fluid being measured, all measured at the same temperature
![Intrinsic Viscosity η The ratio of a solutions specific viscosity to the concentration Intrinsic Viscosity ([η]): The ratio of a solution’s specific viscosity to the concentration](https://slidetodoc.com/presentation_image/3a326d1486ac253754d25ec46df1095a/image-19.jpg)
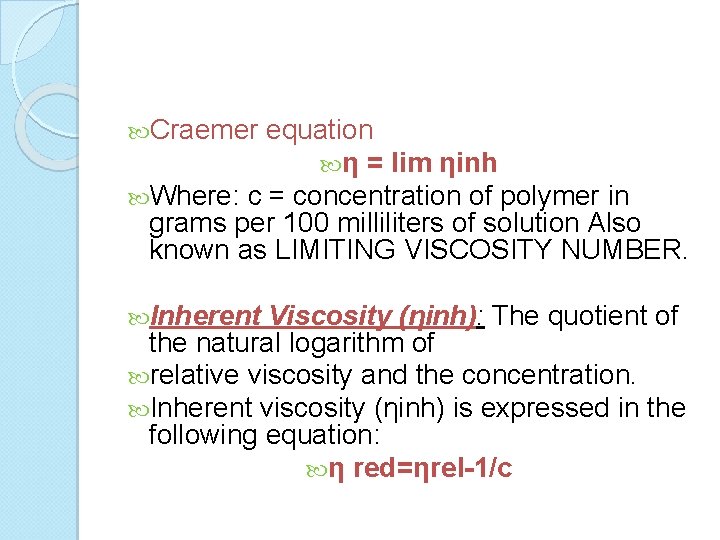
Intrinsic Viscosity ([η]): The ratio of a solution’s specific viscosity to the concentration of the solute, extrapolated to zero concentration. Intrinsic viscosity reflects the capability of a polymer in solution to enhance the viscosity of the solution. The viscosity behavior of macromolecular substances in solution is one of the most frequently used approaches for characterization. The intrinsic viscosity number is defined as the limiting value of the specific viscosity/concentration ratio at zero concentration. Intrinsic viscosity is determined by measuring the relative viscosity at several different concentrations and then extrapolating the specific viscosity to zero concentration. The variation of the viscosity number with concentration depends on the type of molecule as well as the solvent. In general, the intrinsic viscosity of linear macromolecular substances is related to the molecular weight or degree of polymerization. With linear macromolecules, viscosity number measurements can provide a method for the rapid determination of molecular weight when the relationship between viscosity and molecular weight has been established. Intrinsic viscosity is calculated by determining the reduced viscosity (ηsp/c) and extrapolating to infinite dilution. Huggins equation c→ 0 η = ηred

Craemer equation η = lim ηinh Where: c = concentration of polymer in grams per 100 milliliters of solution Also known as LIMITING VISCOSITY NUMBER. Inherent Viscosity (ηinh): The quotient of the natural logarithm of relative viscosity and the concentration. Inherent viscosity (ηinh) is expressed in the following equation: η red=ηrel-1/c

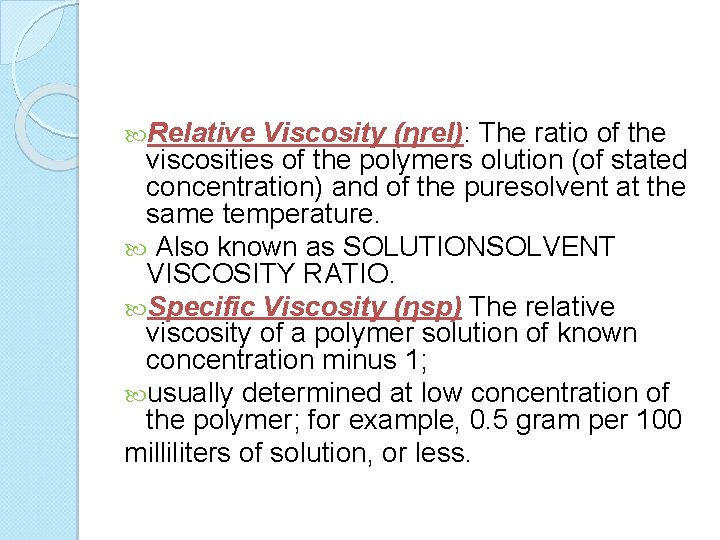
Relative Viscosity (ηrel): The ratio of the viscosities of the polymers olution (of stated concentration) and of the puresolvent at the same temperature. Also known as SOLUTIONSOLVENT VISCOSITY RATIO. Specific Viscosity (ηsp) The relative viscosity of a polymer solution of known concentration minus 1; usually determined at low concentration of the polymer; for example, 0. 5 gram per 100 milliliters of solution, or less.

Thank you The next part will be Electrical conductance

Conductivity PART 11 14 -4 -2013

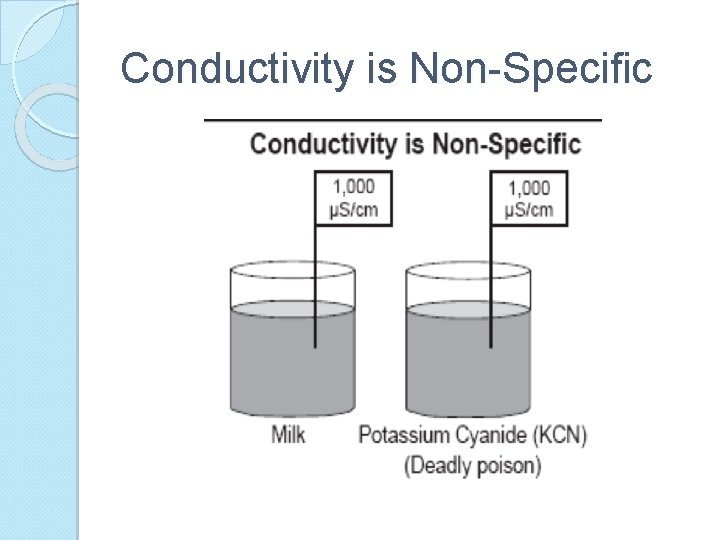
Conductivity A measure of how well a solution conducts electricity ◦ Water with absolutely no impurities (does not exist) Conducts electricity very poorly ◦ Impurities in water increase conductivity So, when measure conductivity of water can estimate the degree of impurities

Conductivity The current is carried by dissolved ions The ability of an ion to carry current is a function of: ◦ Ions charge (more charge, more current) ◦ Ions mass or size (larger ions, conduct less)

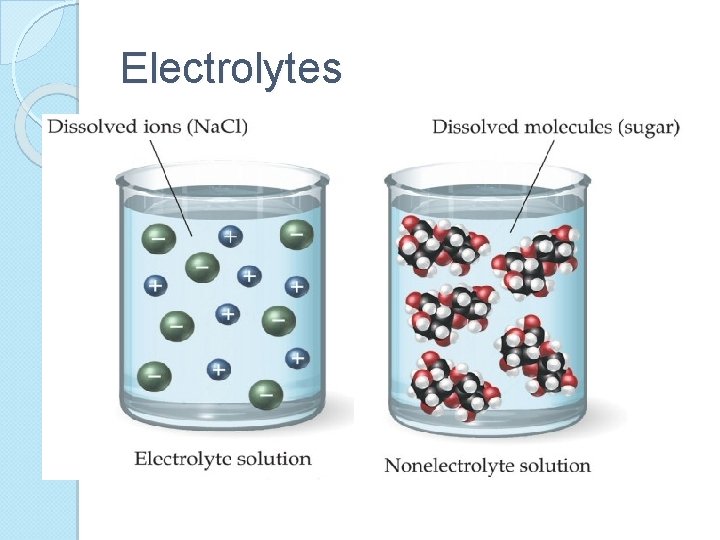
Electrolytes ◦ Substances whose aqueous solution is a conductor of electricity Strong electrolytes ◦ All the electrolyte molecules are dissociated into ions Weak electrolytes ◦ A small percentage of the molecules are dissociated into ions Nonelectrolytes ◦ None of the molecules are dissociated into ions

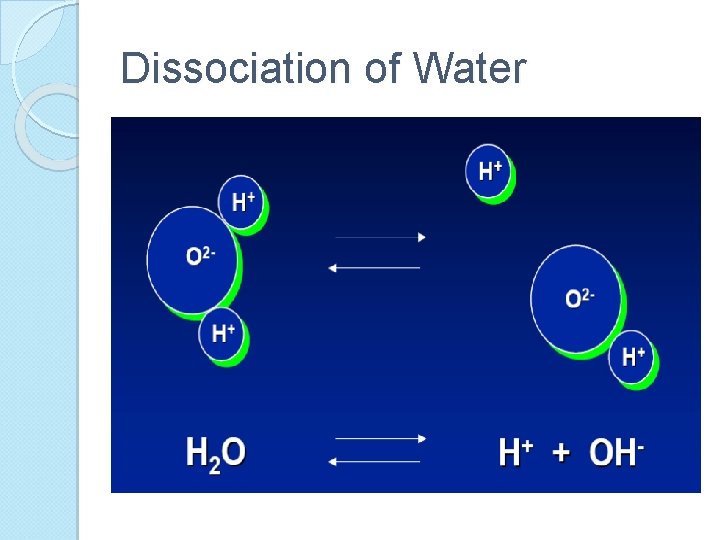
Dissociation of Water

Electrolytes

Strength of Solutions Conductivity of various solutions Conductivity of a solution is proportional to its ion concentration ◦ Since charge on ions in solution facilities the conductance of electrical current

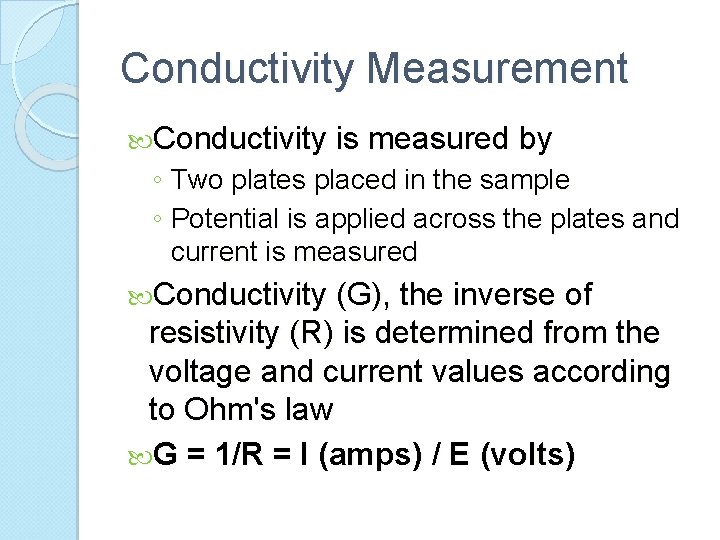
Conductivity Measurement Conductivity is measured by ◦ Two plates placed in the sample ◦ Potential is applied across the plates and current is measured Conductivity (G), the inverse of resistivity (R) is determined from the voltage and current values according to Ohm's law G = 1/R = I (amps) / E (volts)

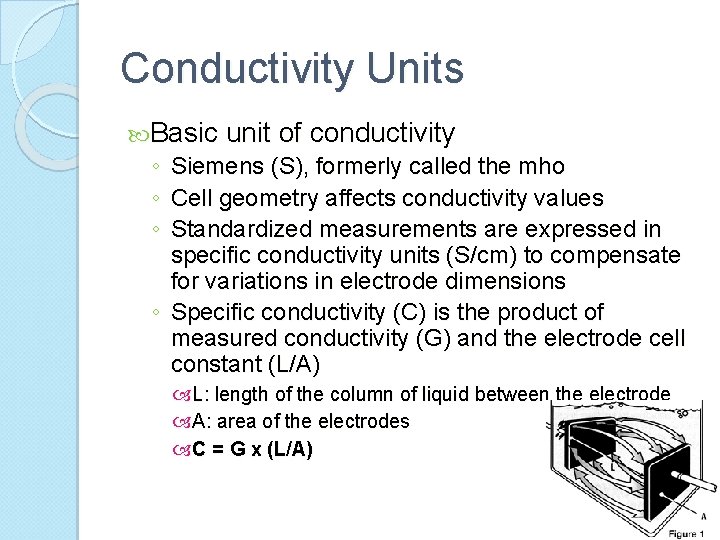
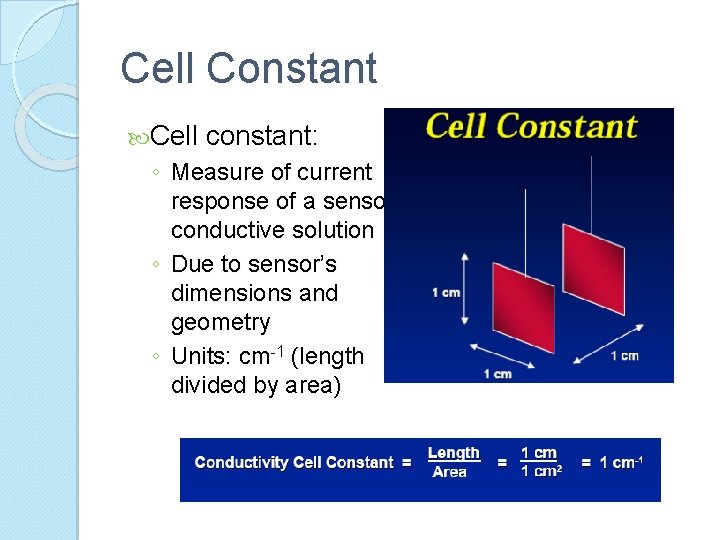
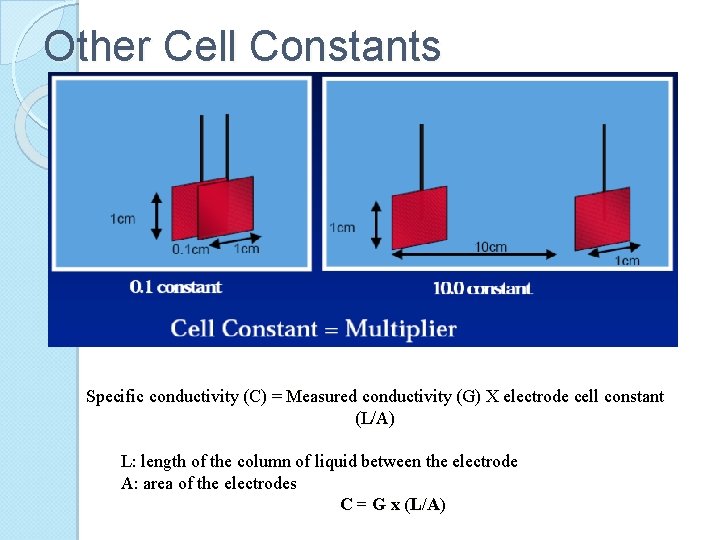
Conductivity Units Basic unit of conductivity ◦ Siemens (S), formerly called the mho ◦ Cell geometry affects conductivity values ◦ Standardized measurements are expressed in specific conductivity units (S/cm) to compensate for variations in electrode dimensions ◦ Specific conductivity (C) is the product of measured conductivity (G) and the electrode cell constant (L/A) L: length of the column of liquid between the electrode A: area of the electrodes C = G x (L/A)

Conductivity of Common Solutions Solution ◦ ◦ ◦ Absolute pure water Power plant boiler water Good city water Ocean water 31% HNO 3 Conductivity ◦ ◦ ◦ 0. 055 µS/cm 1. 0 µS/cm 53 m. S/cm 865 m. S/cm

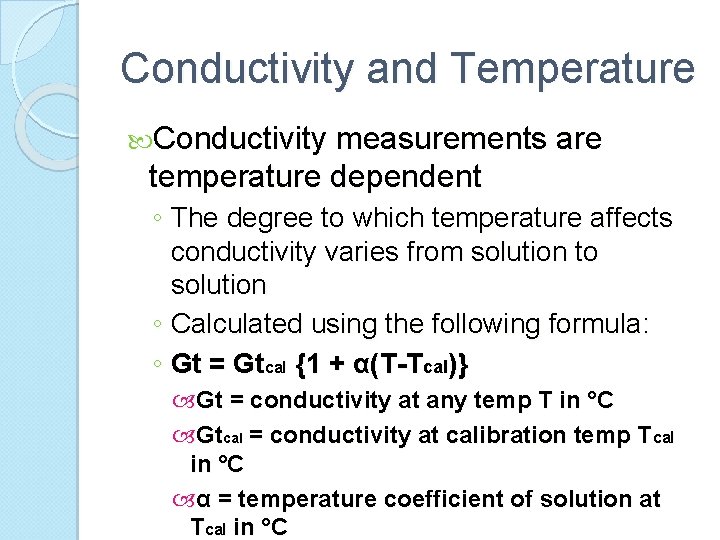
Conductivity and Temperature Conductivity measurements are temperature dependent ◦ The degree to which temperature affects conductivity varies from solution to solution ◦ Calculated using the following formula: ◦ Gt = Gtcal {1 + α(T-Tcal)} Gt = conductivity at any temp T in °C Gtcal = conductivity at calibration temp Tcal in °C α = temperature coefficient of solution at Tcal in °C

Conductivity and Temperature Common alphas (α ) are listed in tables To determine that a of other solutions ◦ Measure conductivity at a range of temperatures ◦ Graph the change in conductivity versus the change in temperature ◦ Divide the slope of the graph by Gtcal to get α

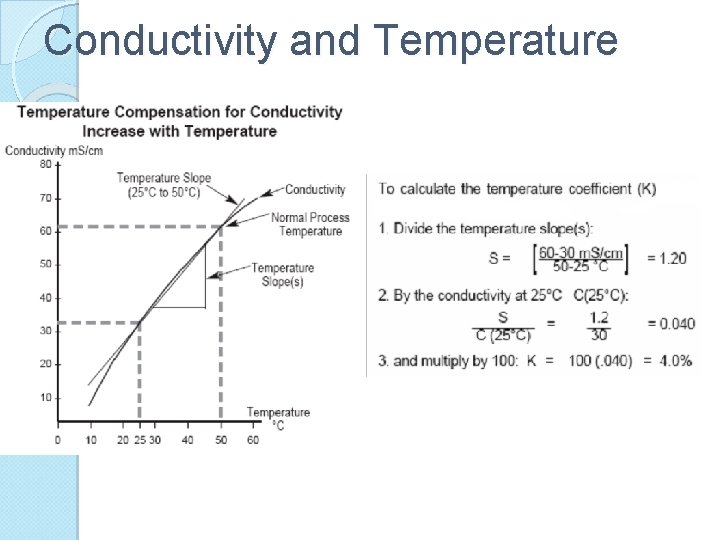
Conductivity and Temperature Conductivity of a solution typically increases with temp In moderately and high conductive solutions, this increase can be compensated for ◦ Using a linear equation involving temp coefficient (K) ◦ K= Percent increase in conductivity per degree centigrade Temp coefficient for the following electrolytes generally fall in the ranges below

Conductivity and Temperature

Conductivity and Temperature

Conductivity is Non-Specific

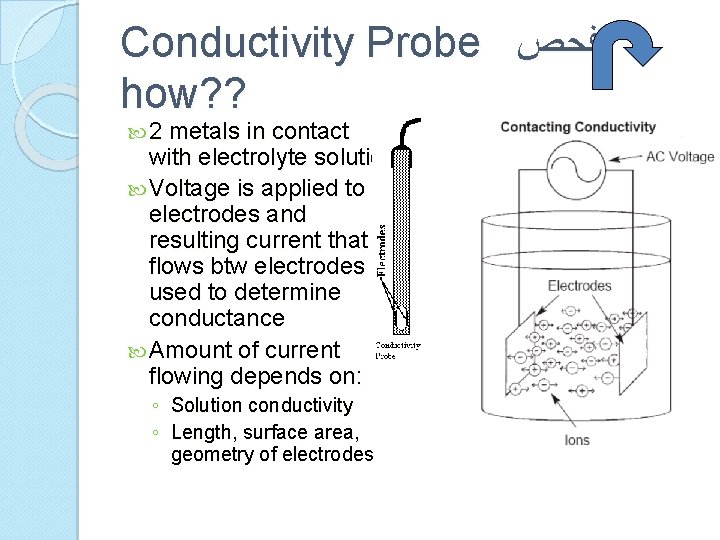
Conductivity Probe ﻓﺤﺺ how? ? 2 metals in contact with electrolyte solution Voltage is applied to electrodes and resulting current that flows btw electrodes is used to determine conductance Amount of current flowing depends on: ◦ Solution conductivity ◦ Length, surface area, geometry of electrodes

Conductivity Probe Apply an AC Voltage to Two Electrodes of Exact Dimensions Acids, Bases and Salts (Na. Cl) Dissolve in Solution and Act as Current Carriers Current Flow is Directly Proportional to the Total Dissolved Solids in Solution Physical Dimensions of a Conductivity Electrode are Referred to as the Cell Constant is Length/Area Relationship ◦ Distance Between Plates = 1. 0 cm ◦ Area of Each Plate = 1. 0 cm x 1. 0 cm ◦ Cell Constant = 1. 0 cm-1

Cell Constant Cell constant: ◦ Measure of current response of a sensor conductive solution ◦ Due to sensor’s dimensions and geometry ◦ Units: cm-1 (length divided by area)

Other Cell Constants Specific conductivity (C) = Measured conductivity (G) X electrode cell constant (L/A) L: length of the column of liquid between the electrode A: area of the electrodes C = G x (L/A)


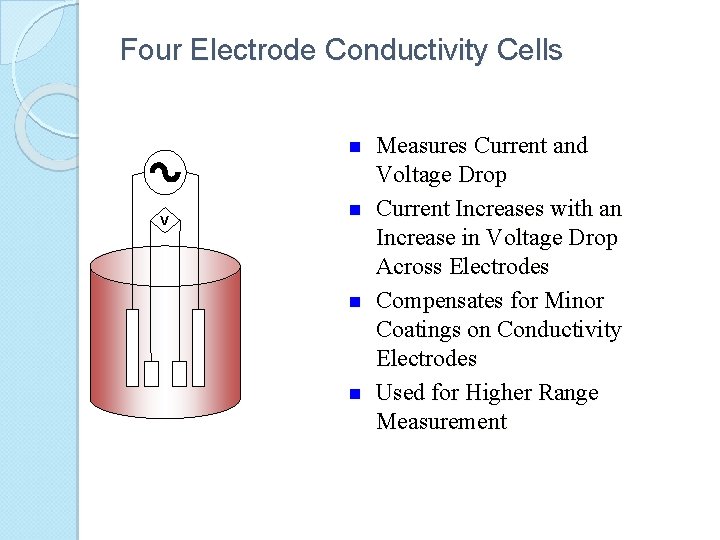
Four Electrode Conductivity Cells n V n n n Measures Current and Voltage Drop Current Increases with an Increase in Voltage Drop Across Electrodes Compensates for Minor Coatings on Conductivity Electrodes Used for Higher Range Measurement

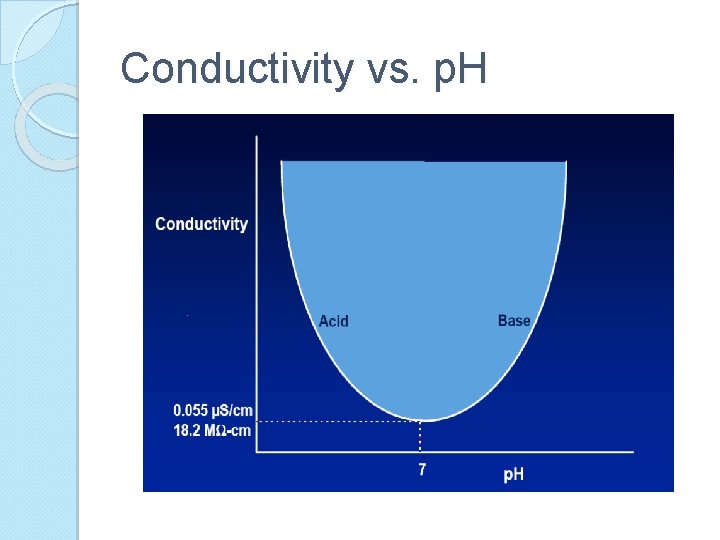
Conductivity vs. p. H

Total Dissolved Solids Total dissolved solids (TDS): ◦ Measure of total amount of all materials that are dissolved in water ◦ These materials, both natural and made by humans Inorganic solids, with a minor amount of organic material ◦ Depending on the type of water, TDS can vary ◦ Seawater contains 3. 5% (35, 000 mg/L) TDS ◦ EPA Secondary Drinking Water Standards recommends that the TDS concentrations in drinking water not exceed 0. 05% (500 mg/L), based on taste and aesthetics

Measuring TDS With Conductivity Method TDS = 0. 7 σ ◦ σ = conductivity (μs/cm) Electrical conductivity of water is directly related to the concentration of dissolved solids in the water ◦ Ions from the dissolved solids in water influence the ability of that water to conduct an electrical current, which can be measured using a conductivity meter ◦ When correlated with laboratory TDS measurements, electrical conductivity can provide an accurate estimate of the TDS concentration

Hardness Waters that contain a significant concentration of dissolved minerals like calcium, magnesium, strontium, iron, and manganese, are called "hard“ ◦ Because it takes a large amount of soap to produce a lather or foam with these waters. Total hardness is expressed as mg/L of calcium carbonate because calcium (Ca) and carbonate (CO 3) are dominant ions in most hard waters The following table gives concentration of Ca. CO 3 dissolved in water by its degree of hardness. Degree of Hardness mg/L as Ca. CO 3 Soft 0 -60 Moderately Hard 60 -120 Hard 120 -180

Sample Measurements Rinse the conductivity cell sensing element with DI water between sample Dip cell up and down in sample 2 -3 times to completely wet surface Allow air bubbles to escape from conductivity cell side holes by tilting cell slightly It is important to control sample temp ◦ Since reading will continue to drift until the temp has stabilized

Storage 1. Best to store conductivity probe so that electrodes are immersed in DI water 2. Can also store dry ◦ Before use: ◦ Probe should be soaked in DI water for 510 minutes ◦ To assure complete wetting of the electrodes

Cleaning 1. For most applications, a hot solution of water with mild lab detergent can be used for cleaning 2. Dilute 1% nitric acid may be used followed by DI water rinsing