Chapter Introduction Lesson 1 Solids Liquids and Gases




























































- Slides: 89

Chapter Introduction Lesson 1 Solids, Liquids, and Gases Lesson 2 Changes in State Lesson 3 The Behavior of Gases Chapter Wrap-Up

Solids, Liquids, and Gases • How do particles move in solids, liquids, and gases? • How are the forces between particles different in solids, liquids, and gases?

Solids, Liquids, and Gases • solid • gas • liquid • vapor • viscosity • surface tension

Describing Matter Two factors determine the state of matter: • the motion of the particles of the matter • the forces between the particles of the matter

Describing Matter (cont. ) Regardless of how close particles are to each other, they all have random motion; movement in all directions and at different speeds. Collisions of particles usually change the speed and direction of the particles’ movements.

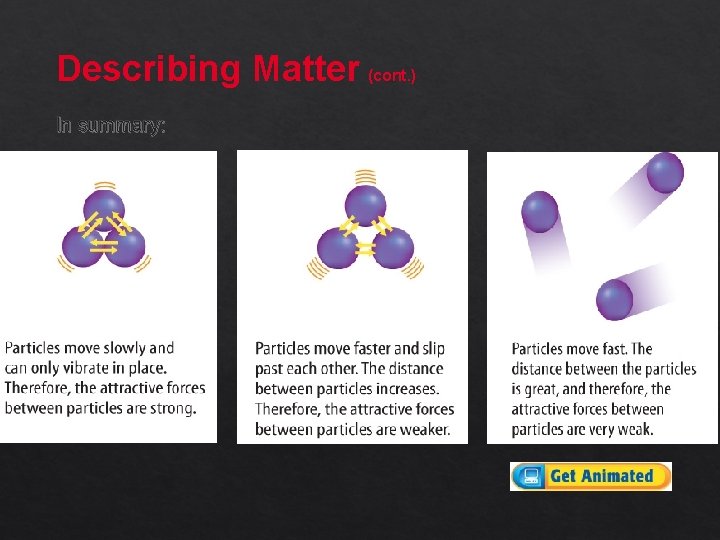
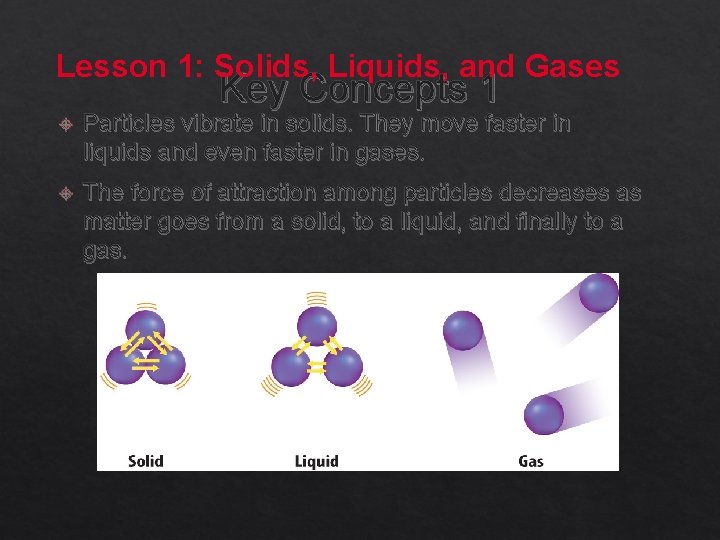
Describing Matter (cont. ) In some matter, the particles move slowly. The particles vibrate in place. The attractive forces between the particle are strong.

Describing Matter (cont. ) In other matter, the particles move faster and slip past each other. The distance between the particles increases. The attractive forces between the particles are weaker.

Describing Matter (cont. ) In other matter, the particles move very fast. The distance between the particles is great. The attractive forces between the particles are very

Describing Matter (cont. ) In summary:

Describing Matter (cont. ) As the motion of the particles slows, the particles move closer. The attractive forces become stronger. As the motion of the particles increases, the particles move farther apart. The attractive forces become weaker.

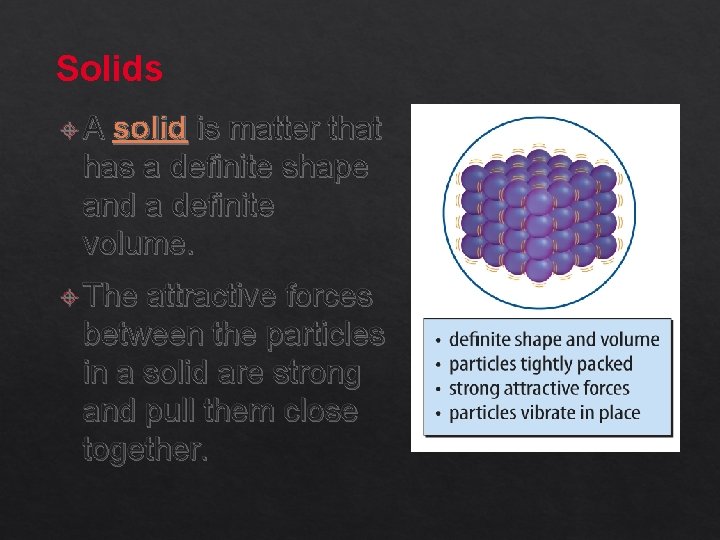
Solids A solid is matter that has a definite shape and a definite volume. The attractive forces between the particles in a solid are strong and pull them close together.

Solids (cont. ) Describe the movement of particles in a solid and the forces between them.

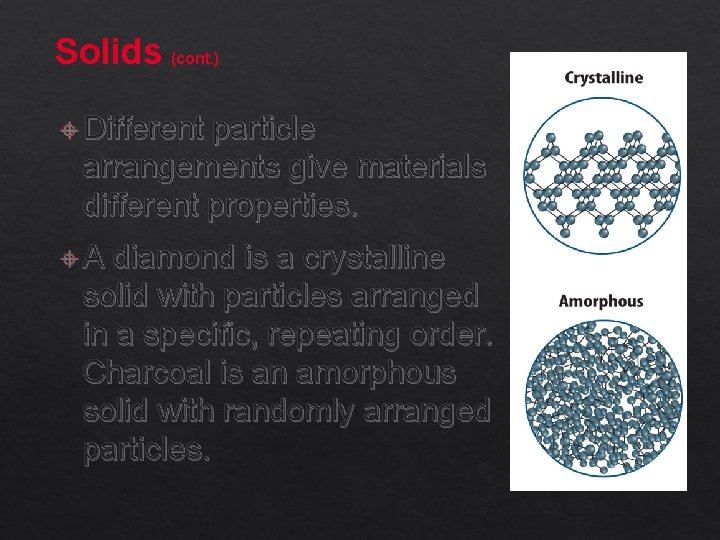
Solids (cont. ) Different particle arrangements give materials different properties. A diamond is a crystalline solid with particles arranged in a specific, repeating order. Charcoal is an amorphous solid with randomly arranged particles.

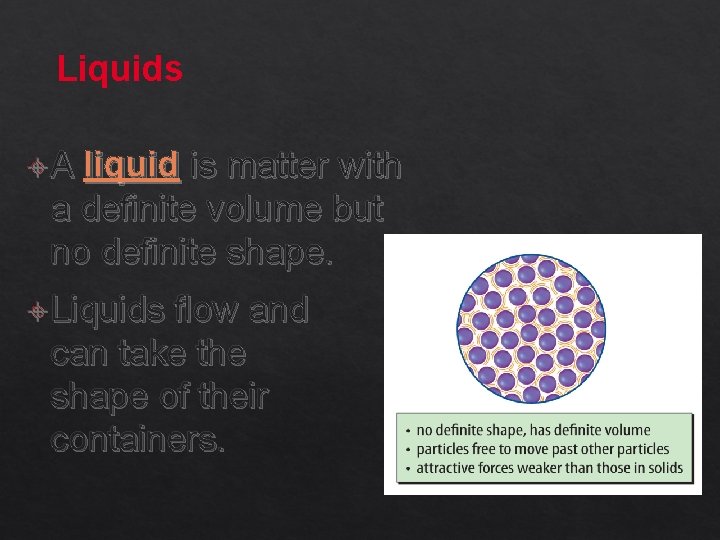
Liquids A liquid is matter with a definite volume but no definite shape. Liquids flow and can take the shape of their containers.

Liquids (cont. ) The particle motion in a liquid is faster than the particle motion in a solid.

Liquids (cont. ) Viscosity is a measurement of a liquid’s resistance to flow. Scott Thomas/Getty Images Dr. Parvinder Sethi

Liquids (cont. ) Molecules Lesson 1 -3 at the surface of a liquid have surface tension, the uneven forces acting on the particles on the surface of a liquid. The surface tension of water enables certain insects to walk on the surface of a lake.

Liquids (cont. ) Lesson 1 -2 Describe the movement of particles in a liquid and the forces between them.

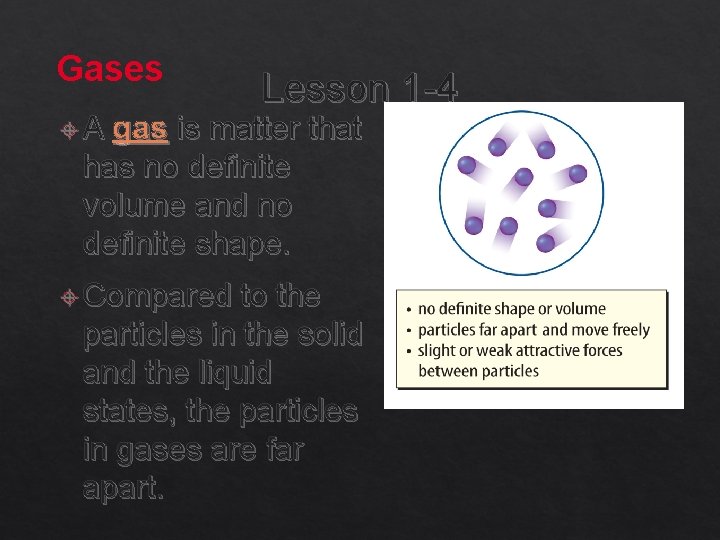
Gases A Lesson 1 -4 gas is matter that has no definite volume and no definite shape. Compared to the particles in the solid and the liquid states, the particles in gases are far apart.

Gases (cont. ) In Lesson 1 -4 a gas, the forces of attraction between the particles are not strong enough to keep the particles close together. Because the particles in gas are moving quickly, the distance between particles increases, and the attractive forces between particles decreases. The gas state of a substance that is normally a solid or a liquid at room temperature is called vapor.

Gases (cont. ) Lesson 1 -2 How do particles move and interact in a gas?

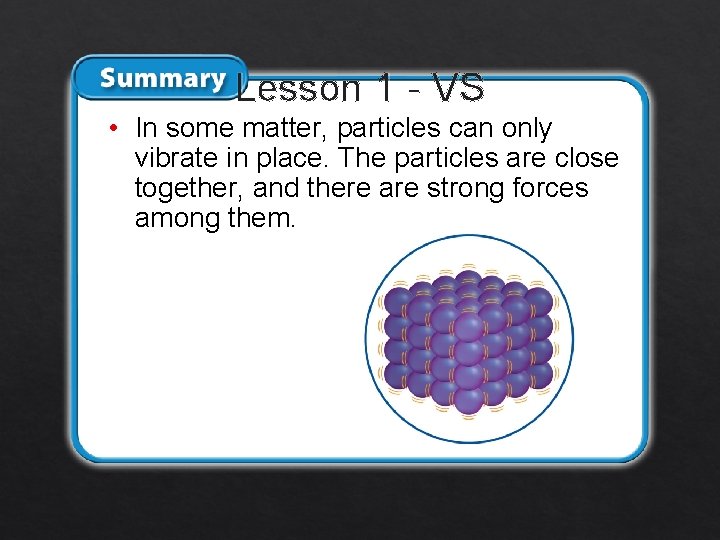
Lesson 1 - VS • In some matter, particles can only vibrate in place. The particles are close together, and there are strong forces among them.

Lesson 1 - VS • In other matter, the particles are far enough apart that particles can flow past other particles. The forces among these particles are weaker than those shown above.

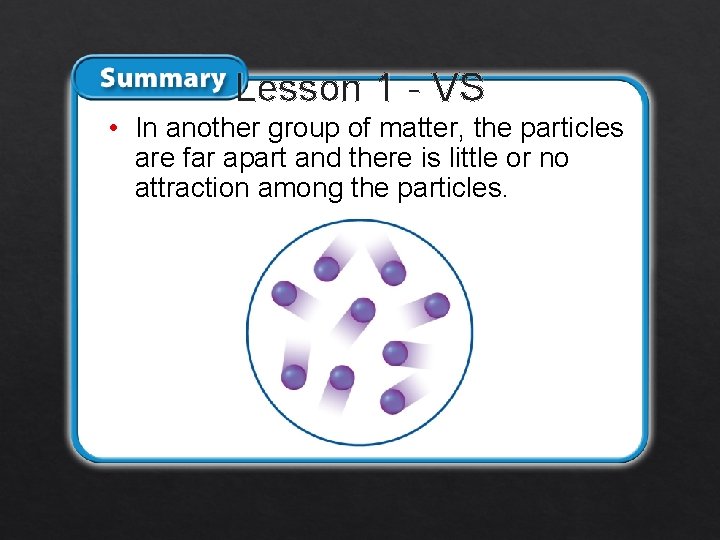
Lesson 1 - VS • In another group of matter, the particles are far apart and there is little or no attraction among the particles.

Lesson 1 – LR 1 Which describes matter with a definite volume but no definite shape? A. solid B. plasma C. liquid D. gas

Lesson 1 – LR 2 Compared to a liquid, which best describes the particles of a gas? A. closer together B. farther apart C. slower moving D. tightly packed

Lesson 1 – LR 3 Which term refers to the gas state of a substance that is a solid at room temperature? A. plasma B. surface tension C. vapor D. viscosity

Changes in State • How is temperature related to particle motion? • How are temperature and thermal energy different? • What happens to thermal energy when matter changes from one state to another?

Changes in State • kinetic energy • evaporation • temperature • condensation • thermal energy • sublimation • vaporization • deposition

Kinetic and Potential Energy Lesson 2 -1 Particles that make up matter have kinetic energy, the energy an object has due its motion. The faster particles move, the more kinetic energy they have.

Kinetic and Potential Energy (cont. ) Lesson 2 -1 Temperature is a measure of the average kinetic energy of all the particles in an object. Within a given substance, a temperature increase means that the particles, on average, are moving at greater speeds.

Kinetic and Potential Energy (cont. ) Lesson 2 -1 How is temperature related to particle motion?

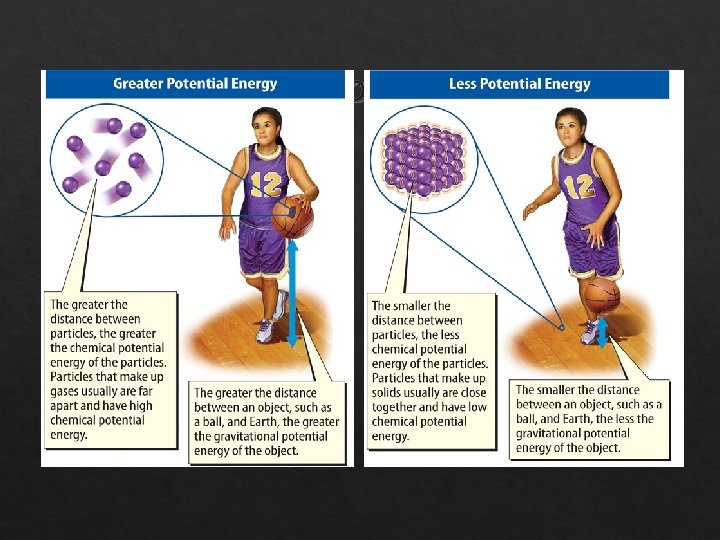
Kinetic and Potential Energy (cont. ) Lesson 2 -1 Potential energy of particles typically increases as the particle get farther apart. The farther an object is from Earth’s surface, the greater the gravitational potential energy.

Lesson 2 -1

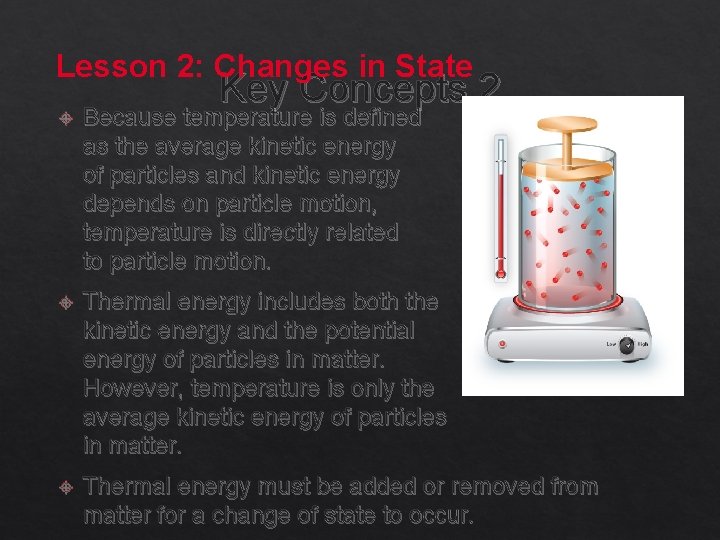
Thermal Energy Lesson 2 -2 Thermal energy is the total potential and kinetic energy of an object. You can change an object’s state of matter by adding or removing thermal energy. If enough thermal energy is added or removed from an object, a change of state can occur.

Thermal Energy (cont. ) Lesson 2 -2 How do thermal energy and temperature differ?

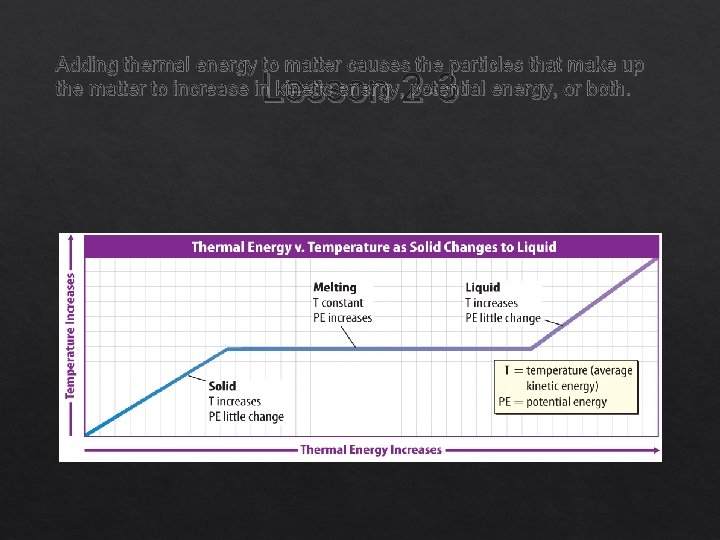
Solid to Liquid or Liquid to Solid Lesson 2 -3 To change matter from a solid to a liquid, thermal energy must be added. Once a solid reaches the melting point, additional thermal energy is used by the particles to overcome their attractive forces, the particles move farther apart and potential energy increases.

Adding thermal energy to matter causes the particles that make up the matter to increase in kinetic energy, potential energy, or both. Lesson 2 -3

Solid to Liquid or Liquid to Solid (cont. ) Lesson 2 -3 Freezing is a process that is the opposite of melting. The temperature at which matter changes from the liquid state to the solid state is its freezing point.

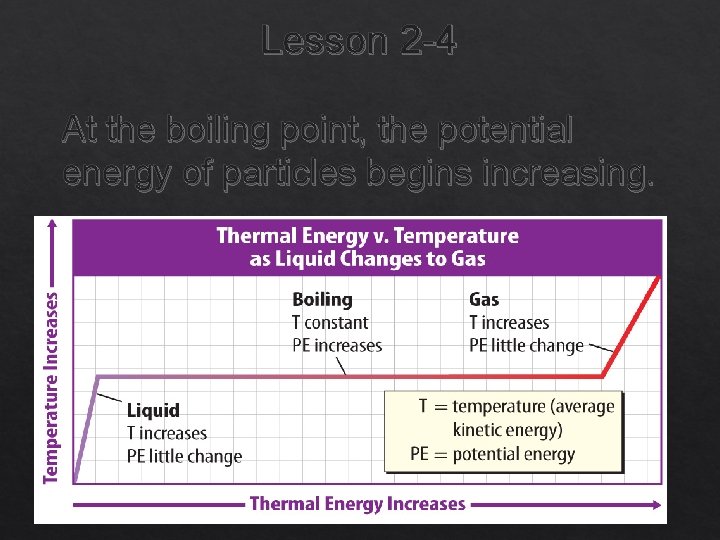
Liquid to Gas or Gas to Liquid Lesson 2 -4 The change in state of a liquid into a gas is vaporization. Vaporization that occurs within a liquid is called boiling and the temperature at which boiling occurs in a liquid is called its boiling point.

Lesson 2 -4 At the boiling point, the potential energy of particles begins increasing.

Liquid to Gas or Gas to Liquid (cont. ) Lesson 2 -4 Evaporation is vaporization that occurs only at the surface of a liquid. evaporation from Latin evaporare, means “disperse in steam or vapor”

Liquid to Gas or Gas to Liquid (cont. ) Lesson 2 -4 When a gas loses enough thermal energy, the gas changes to a liquid, or condenses. The change of state from a gas to a liquid is called condensation.

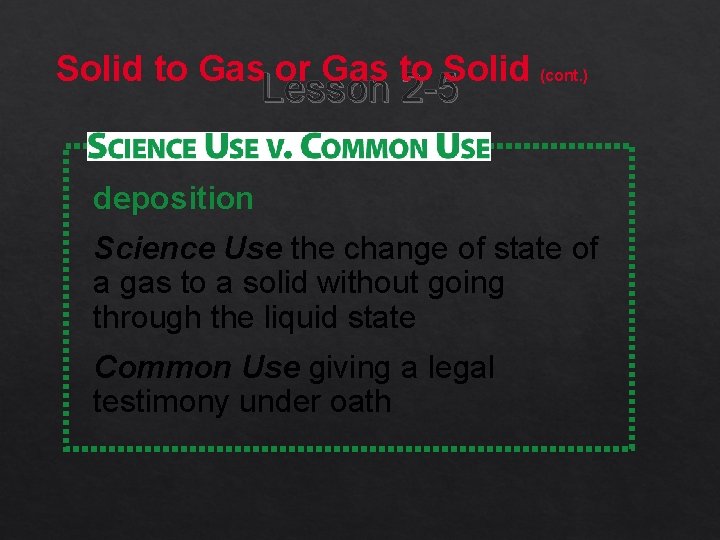
Solid to Gas or Gas to Solid Lesson 2 -5 Sublimation is the change of state from a solid to a gas without going through the liquid state. Deposition is the change of state of a gas to a solid without going through the liquid state.

Solid to Gas or Gas to Solid (cont. ) Lesson 2 -5 deposition Science Use the change of state of a gas to a solid without going through the liquid state Common Use giving a legal testimony under oath

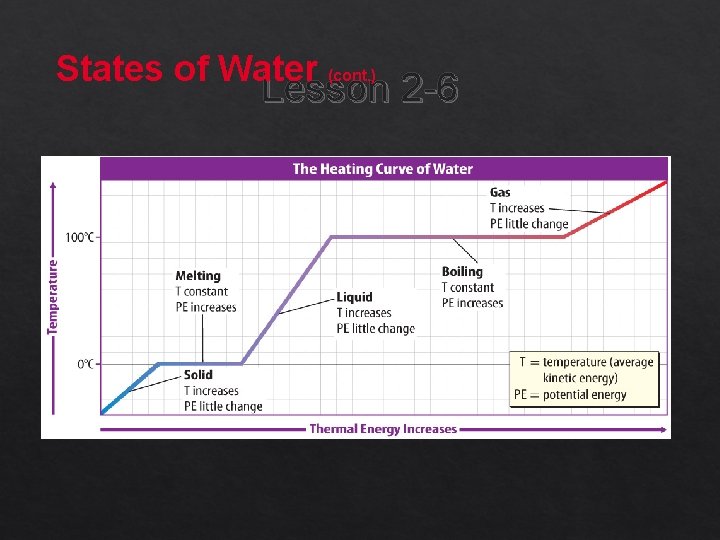
States of Water Lesson 2 -6 Water is the only substance that exists naturally as a solid, a liquid, and a gas on Earth. At 0°C, water molecules vibrate so rapidly that they begin to move out of their places, the particles overcome their attractive forces, and melting occurs.

States of Water (cont. ) Lesson 2 -6 When water reaches 100°C, the boiling point, liquid water begins to change to water vapor. Cooling water vapor changes the gas to a liquid, and cooling the water further changes it to ice.

States of Water (cont. ) Lesson 2 -6

States of Water (cont. ) Lesson 2 -2 Describe the changes in thermal energy as water goes from a solid to a liquid.

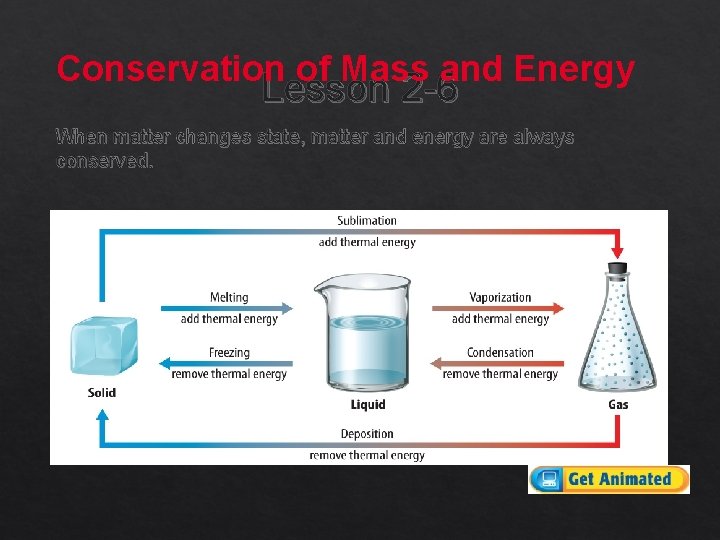
Conservation of Mass and Energy Lesson 2 -6 When matter changes state, matter and energy are always conserved.

Lesson 2 - VS • All matter has thermal energy, and thermal energy is the sum of potential and kinetic energy. • When thermal energy is added to a liquid, vaporization can occur. • When enough thermal energy is removed from matter, a change of state can occur.

Lesson 2 – LR 1 Which term refers to the average kinetic energy of all the particles in an object? A. thermal energy B. temperature C. sublimation D. evaporation

Lesson 2 – LR 2 Which substance exists naturally on Earth as a solid, liquid and gas? A. carbon B. carbon dioxide C. salt D. water

Lesson 2 – LR 3 Which term refers to the change of state of a gas to a solid without going through the liquid state? A. sublimation B. evaporation C. deposition D. condensation

Lesson 2 - Now Do you agree or disagree? 3. Particles of matter have both potential energy and kinetic energy. 4. When a solid melts, thermal energy is removed from the solid.

Lesson 3 Reading Guide - KC The Behavior of Gases • How does the kinetic molecular theory describe the behavior of a gas? • How are temperature, pressure, and volume related in Boyle’s law? • How is Boyle’s law different from Charles’s law?

The Behavior of Gases • kinetic molecular theory • pressure • Boyle’s Law • Charles’s Law

Understanding Gas Behavior Lesson 3 -1 The kinetic molecular theory states that the particles in matter collide with other particles, other objects, and the walls of their container; and when particles collide, no energy is lost.

Understanding Gas Behavior (cont. ) Lesson 3 -1 How does kinetic molecular theory describe the behavior of a gas?

What is pressure? Pressure Lesson 3 -2 is the amount of force applied per unit of area. The empty spaces between particles makes gases compressible. pressure from Latin pressura, means “to press”

Pressure and Volume Lesson 3 -3 When the volume of a container holding gas is greater, the additional space results in fewer collisions and pressure is less.

Boyle’s Law Lesson 3 -4 Boyle’s law states that pressure of a gas increases if the volume decreases and pressure of a gas decreases if the volume increases, when temperature is constant. What is the relationship between pressure and volume of a gas if temperature is constant?

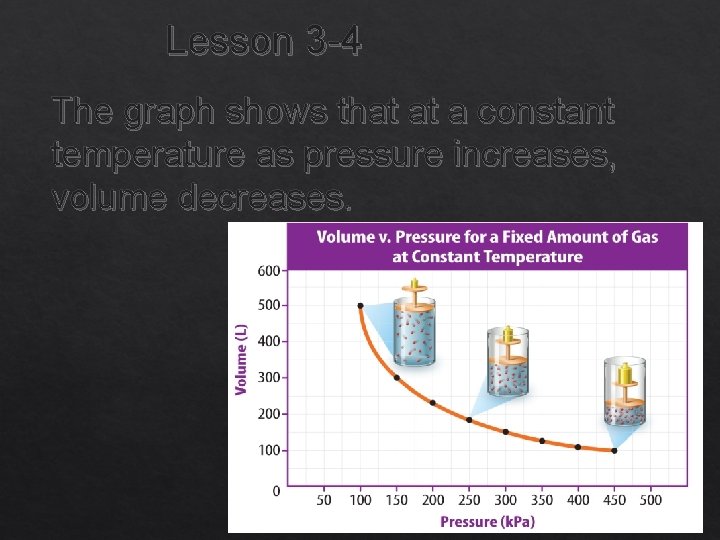
Lesson 3 -4 The graph shows that at a constant temperature as pressure increases, volume decreases.

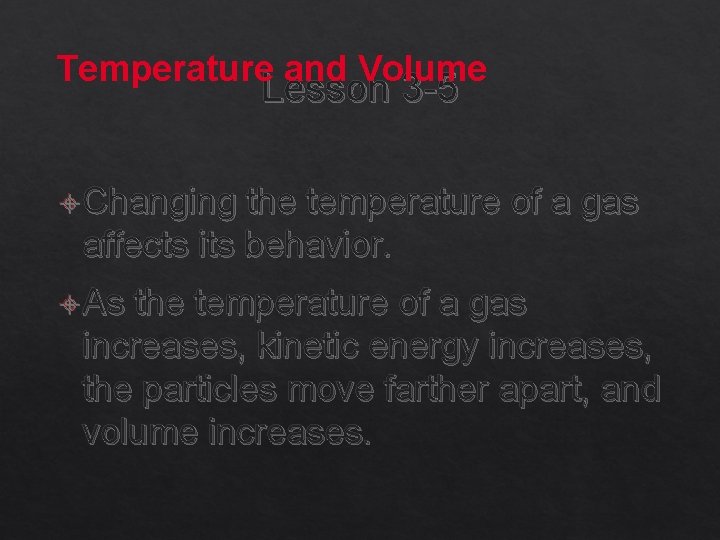
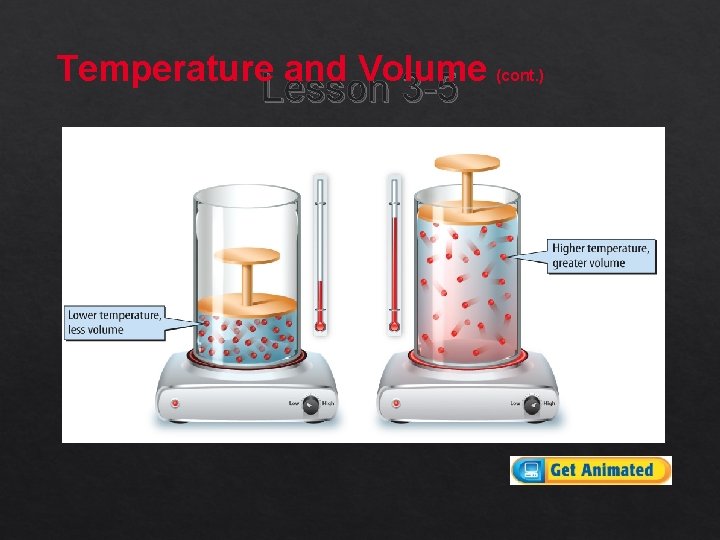
Temperature and Volume Lesson 3 -5 Changing the temperature of a gas affects its behavior. As the temperature of a gas increases, kinetic energy increases, the particles move farther apart, and volume increases.

Temperature and Volume (cont. ) Lesson 3 -5

Charles’s Law Lesson 3 -6 Charles’s law states that the volume of a gas increases with increasing temperature, if the pressure is constant. How is Boyle’s law different from Charles’s law?

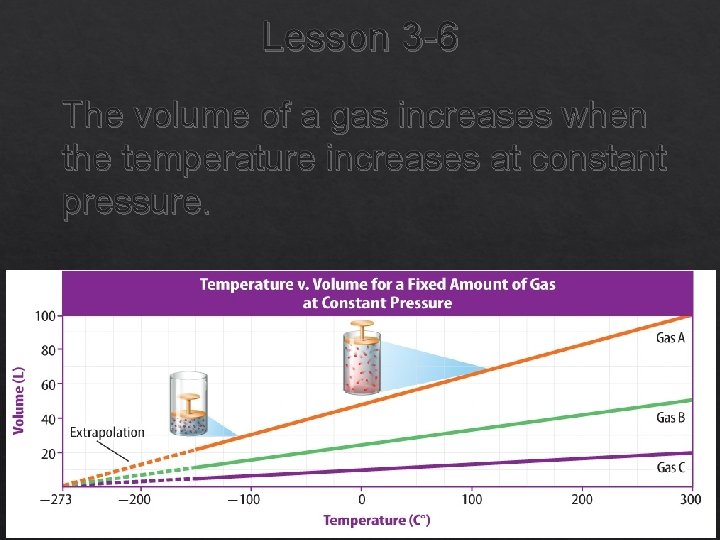
Lesson 3 -6 The volume of a gas increases when the temperature increases at constant pressure.

Charles’s Law (cont. ) Lesson 3 -6 What factors must be constant in Boyle’s law and Charles’s law?

Lesson 3 - VS • The explanation of particle behavior in solids, liquids, and gases is based on the kinetic molecular theory.

Lesson 3 - VS • As pressure in a gas increases, the volume of the gas decreases when at constant temperature.

Lesson 3 - VS • At constant pressure, as the temperature of a gas increases, the volume also increases.

Lesson 3 – LR 1 Which states that pressure of a gas increases if the volume decreases? A. Boyle’s law B. Charles’s law C. kinetic molecular theory D. law of thermal energy

Lesson 3 – LR 2 As the temperature of a gas increases, what happens to its kinetic energy? A. decreases B. increases C. remains unchanged D. rises and falls

Lesson 3 – LR 3 According to the kinetic molecular theory, when particles collide, what happens to energy? A. no energy is lost B. it increases C. it decreases D. all energy is lost

Lesson 3 - Now Do you agree or disagree? 5. Changes in temperature and pressure affect gas behavior. 6. If the pressure on a gas increases, the volume of the gas also increases.

The BIG Idea As matter changes from one state to another, the distances and the forces between the particles change, and the amount of thermal energy in the matter changes.

Lesson 1: Solids, Liquids, and Gases Key Concepts 1 Particles vibrate in solids. They move faster in liquids and even faster in gases. The force of attraction among particles decreases as matter goes from a solid, to a liquid, and finally to a gas.

Lesson 2: Changes in State Key Concepts 2 Because temperature is defined as the average kinetic energy of particles and kinetic energy depends on particle motion, temperature is directly related to particle motion. Thermal energy includes both the kinetic energy and the potential energy of particles in matter. However, temperature is only the average kinetic energy of particles in matter. Thermal energy must be added or removed from matter for a change of state to occur.

Lesson 3: The Behavior of Gases Key Concepts 3 The kinetic molecular theory states basic assumptions that are used to describe particles and their interactions in gases and other states of matter. Pressure of a gas increases if the volume decreases, and pressure of a gas decreases if the volume increases, when temperature is constant. Boyle’s law describes the behavior of a gas when pressure and volume change at constant temperature. Charles’s law describes the behavior of a gas when temperature and volume change, and pressure is constant.

Chapter Review – MC 1 Which term describes a state of matter that has a definite shape and a definite volume? A. gas B. liquid C. solid D. vapor

Chapter Review – MC 2 Which type of matter has a definite volume and take the shape of its container? A. gas B. liquid C. plasma D. solid

Chapter Review – MC 3 Which term describes vaporization that occurs only at the surface of a liquid? A. condensation B. evaporation C. sublimation D. vaporization

Chapter Review – MC 4 What is the melting point of water? A. 0°C B. 10°C C. 100°C D. 1000°C

Chapter Review – MC 5 Which states that the volume of a gas increases with increasing temperature, if the pressure is constant? A. Boyle’s law B. Charles’s law C. the kinetic molecular theory D. the particle theory of matter

Chapter Review – STP 1 Which type of matter has no definite volume and no definite shape? A. gas B. liquid C. plasma D. solid

Chapter Review – STP 2 Which term refers to a measurement of a liquid’s resistance to flow? A. viscosity B. vapor C. temperature D. surface tension

Chapter Review – STP 3 When matter changes state, matter and what else are always conserved? A. energy B. pressure C. temperature D. volume

Chapter Review – STP 4 Which term describes the energy an object has due to its motion? A. chemical energy B. kinetic energy C. potential energy D. thermal energy

Chapter Review – STP 5 Which term refers to the amount of force applied per unit of area? A. temperature B. pressure C. potential energy D. kinetic energy