Properties of Liquids and the Kinetic Molecular Theory




- Slides: 12


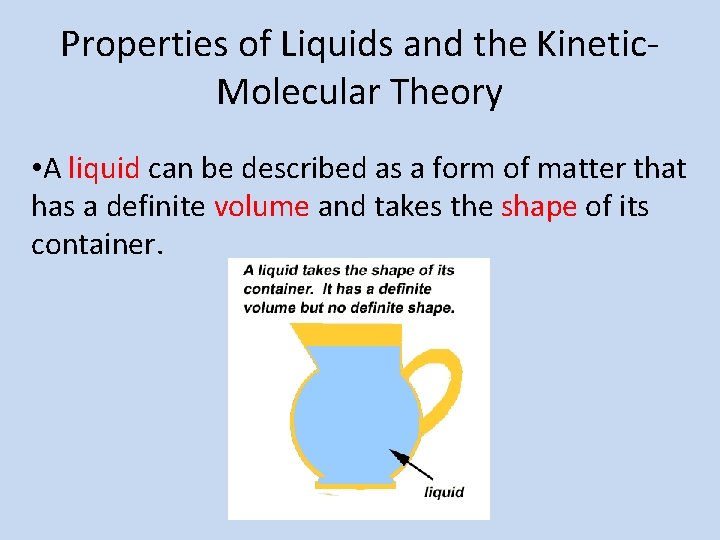
Properties of Liquids and the Kinetic. Molecular Theory • A liquid can be described as a form of matter that has a definite volume and takes the shape of its container.

• The attractive forces between particles in a liquid are more effective than those between particles in a gas. • This attraction is explained by intermolecular forces (dipole-dipole, London dispersion, and hydrogen bonding).

• Like gases, particles in a liquid are in constant motion. • Liquids are more ordered than gases because of the stronger intermolecular forces and lower mobility of particles.

• Relatively High Density – At normal atmospheric pressure, most substances are hundreds of times denser in a liquid state than in a gaseous state. • Due to the close arrangement of liquid particles. • Relative Incompressibility – Liquids are much less compressible than gases because liquid particles are more closely packed together.

• Ability to Diffuse – Any liquid gradually diffuses throughout any other liquid in which in can dissolve. • The constant, random motion of particles causes diffusion in liquids.

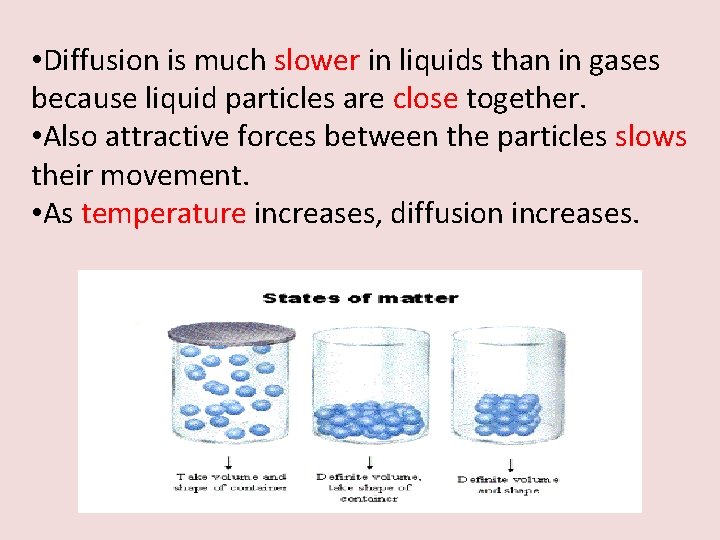
• Diffusion is much slower in liquids than in gases because liquid particles are close together. • Also attractive forces between the particles slows their movement. • As temperature increases, diffusion increases.

• Surface Tension – A property common to all liquids is surface tension, a force that tends to pull adjacent parts of a liquid’s surface together, thereby decreasing surface area to the smallest possible size. • The higher the force of attraction, the higher the surface tension.

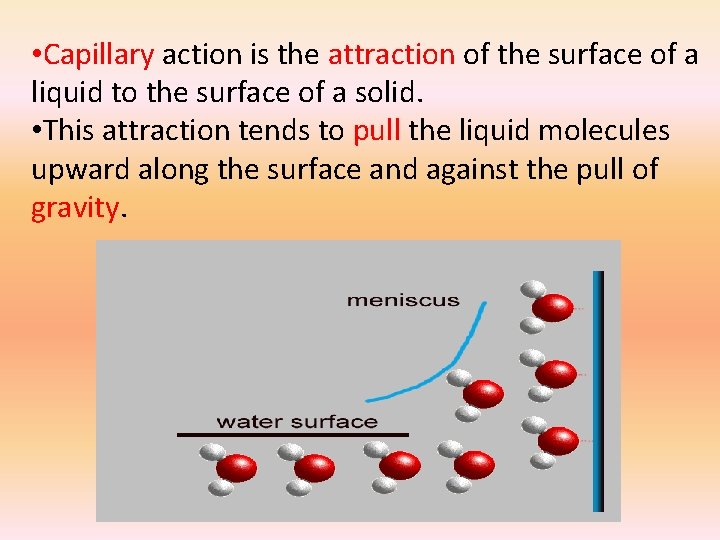
• Capillary action is the attraction of the surface of a liquid to the surface of a solid. • This attraction tends to pull the liquid molecules upward along the surface and against the pull of gravity.

• Vaporization – The process by which a liquid or solid changes to a gas is vaporization. • Evaporation is a form of vaporization. • Evaporation is the process by which particles escape from the surface of a nonboiling liquid and enter the gas state. • Evaporation occurs because the particles of a liquid have different kinetic energies. • Particles with higher kinetic energy move faster and can overcome intermolecular forces. • Evaporation occurs at the surface of a liquid.

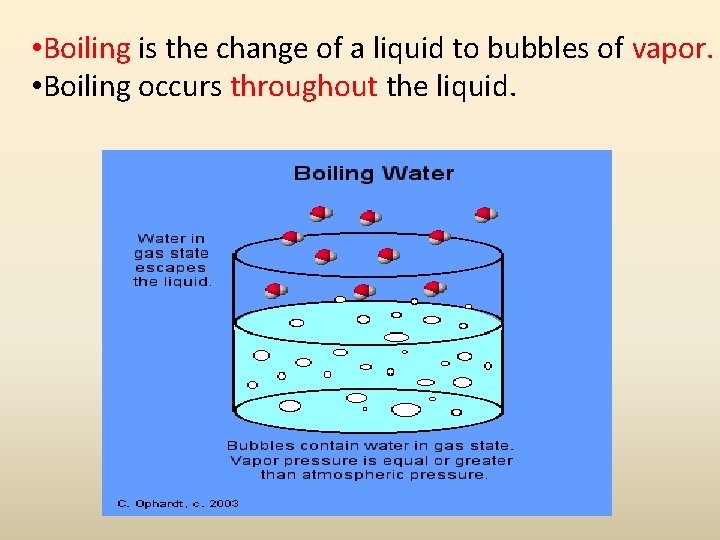
• Boiling is the change of a liquid to bubbles of vapor. • Boiling occurs throughout the liquid.

• The physical change of a liquid to a solid by removal of energy as heat is called freezing or solidification. • When a liquid is cooled, the average kinetic energy of its particles decreases. • When energy is low enough, attractive forces pull the particles in a more orderly arrangement.