MONOPHASIC LIQUID DOSAGE FORMS SOLUTION A solution is





















































- Slides: 63

MONOPHASIC LIQUID DOSAGE FORMS

SOLUTION: “A solution is a liquid-preparation that contains one or more soluble chemical substances dissolved in a specified solvent. ” l Advantages Immediately available for absorption Useful for hygroscopic drugs which must be administered as a solution l Disadvantages Less stable Incompatibility is faster Bacterial contamination l

Solvents used A. Aqueous liquids: Water Purified Water Water for injection Sterile water for injection Bacteriostatic water for injection B. Oils &oily materials: Animals source…pig fat Vegetable Source…corn, cotton Mineral Source…liquid paraffin Synthetics…. ethyl oleate C. Hydroxylated Compounds: Ethanol Propylene Glycol D. Hydro-alcoholic Mixtures E. Modern Vehicles: Dioxalanes Solvent for aerosol sprays Dimethylecetamide

-Vehicle for the internal Use 1. Purified Water 2. Aromatic Water a. Chloroform Water b. Peppermint Water c. Cinnamon Water d. Dill Water - vehicles for external use Isopropyl myristate Liquid Paraffin -vehicles for extraction Isopropyl Alcohol Acetone

Formulation Consideration l 1) Solubility a) p. H b) Cosolvency c) Solubilization d) Complexation e) Hydrotrophy f) Chemical modification of the drug molecule 2) Preservation a) Preservatives b) Antioxidants c) Reducing agents d) Synergists 3) Organoleptic consideration a) Sweetening agents b) Flavoring agents c) Coloring agents d) Viscosity control e) Overall appearance 4) Stability a) Chemical stability b) Physical stability

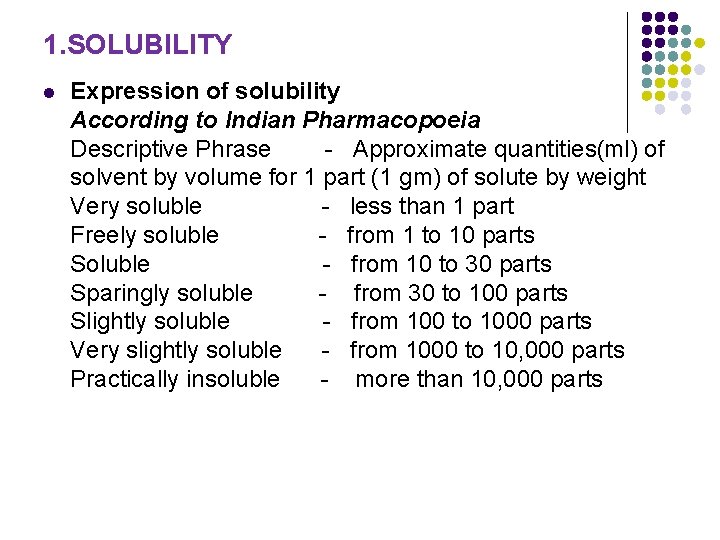
1. SOLUBILITY l Expression of solubility According to Indian Pharmacopoeia Descriptive Phrase - Approximate quantities(ml) of solvent by volume for 1 part (1 gm) of solute by weight Very soluble - less than 1 part Freely soluble - from 1 to 10 parts Soluble - from 10 to 30 parts Sparingly soluble - from 30 to 100 parts Slightly soluble - from 100 to 1000 parts Very slightly soluble - from 1000 to 10, 000 parts Practically insoluble - more than 10, 000 parts

a. p. H A large number of drugs are either weak acids or weak bases. The solubility of these agents can be markedly influenced by the p. H of the environment l Weak Acid p. H = p. Ka + log (ionised ) /(un ionised) p. H = p. Ka + log (base) / (acid) Weak Base p. H=p. Ka +log (unionised) / (ionised) p. H = p. Ka+ log( base )/(acid) b. COSOLVENCY Weak electrolytes and nonpolar molecules frequently have poor water solubility. These types of solutes are more soluble in a mixture of solvents than in one solvent alone. This phenomenon is known as cosolvency.

c. DIELECTRIC CONSTANT One property of a solvent system is its dielectric constant. The dielectric constant of a solvent can be defined as the ratio of the capacitances of a capacitor filled with the solvent and air respectively. d. SOLUBILIZATION spontaneous increase of solubility of a poorly water-soluble solute molecules into an aqueous solution of surface active agents (or surfactants) in which a thermodynamically stable solution is formed.

e. COMPLEXATION Solubility of a compound may be increased by complexing with a complexing agent. e. g. solubility of para amino benzoic acid (PABA) may be increased by complexing with caffeine f. HYDROTROPHY The term hydrotrophy has been used to designate the increase in solubility in water of various substances due to the presence of large amounts of additives. g. CHEMICAL MODIFICATION OF DRUG Poorly soluble drugs are chemically modified into water soluble derivatives.

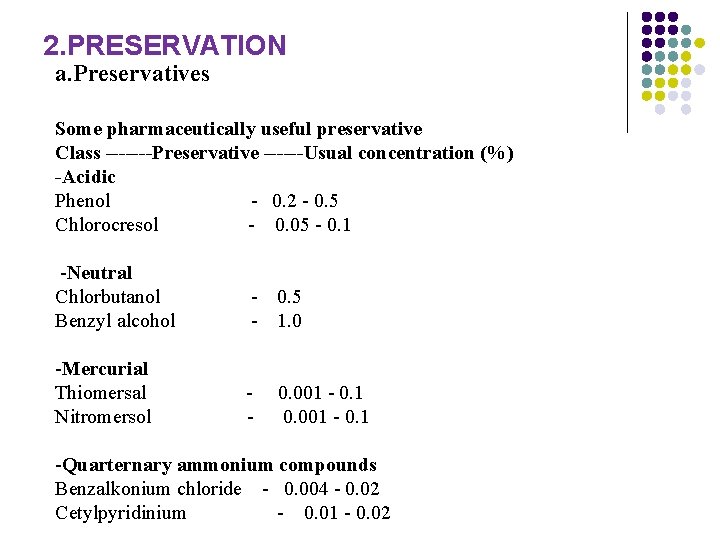
2. PRESERVATION a. Preservatives Some pharmaceutically useful preservative Class -------Preservative ------Usual concentration (%) -Acidic Phenol - 0. 2 - 0. 5 Chlorocresol - 0. 05 - 0. 1 -Neutral Chlorbutanol - 0. 5 Benzyl alcohol - 1. 0 -Mercurial Thiomersal - 0. 001 - 0. 1 Nitromersol - 0. 001 - 0. 1 -Quarternary ammonium compounds Benzalkonium chloride - 0. 004 - 0. 02 Cetylpyridinium - 0. 01 - 0. 02

b. Antioxidants An antioxidant is a molecule that inhibits the oxidation of other molecules c. Reducing agent A reducing agent (also called a reductant or reducer) is the element or compound in a reduction-oxidation reaction that donates an electron to another species however, since the reducer loses an electron we say it is "oxidized". This means that there must be an "oxidizer"; because if any chemical is an electron donor (reducer), another must be an electron recipient (oxidant).

3. ORGANOLEPTIC ADDITIVES a. Colouring Agents l Colouring Agents generally used in case of cosmetic preparations. l The second reasons is to distinguish one product from the products. A third reason is to distinguish the preparation being administered l Classification- 1. natural colouring agents-plant -animal -minerals 2. syntetic -coal tar

1. Natural Colouring Agents: l Plants: l Many plants contain colouring agents which may be extracted. and used as colorant. l Some Examples are: a. Chlorophyll-green b. Annatto seeds-yellow to orange

Animal: - Cochineal: It is an alkaline solution of the soluble Colouring principles caraminic acid of cochineal insects preserved by the addition of. It is very dark purplish red liquid. Minerals: -Mineral colours are termed pigments. -They are used to colour lotions, cosmetics and other preparation for external application. -As they are toxic, their use for internal preparation is forbidden. Ex: Red ferric oxide Yellow Ferric Dioxide

2. Synthetic colouring agents l The synthetic colours are coal tar dyes, because many of them are produced from substance obtained from coal-tar. l The certified colours are classified into three groups: l Group I- F. D. and C. Colours used in foods, drugs and cosmetics. l Groups II- The D. and C. Colour used in drug and Cosmetics. l Group III- The External D. and C. Colour.

b. Flavouring agents The use of flavour is actually a composite sensation of taste, touch, smell, sound and heat. l There are simply four types of tastes - Sweet - Sour - Salty - Bitter. Similarly there are seven basic odours like - Ethereal , camphoraceous , Musky, Floral , Pepperminty , Pungent , Putrid l

Classification of flavouring agents Two Types: 1. Natural and 2. Synthetic 1. Natural a. Fruits (Sweet, Sur and Astringent) Citrus Fruits (Orange, Lemon) b. Seeds (Vanilla, Anise, Nutmeg) 2. synthetics Benzaldehyde - Bitter Almond, Cherry pits Decyl Aldehyde - Citrus Flavours (Orange) Cinnamic aldehyde - Cinnamon type

c. Sweetening agents l l l l Sucrose Sorbitol (Half Sweet than Sucrose) Glycerin Honey Saccharin Sodium (300 -550 times) Cyclametaes (30 times sweeter than sucrose) Aspartame

d. Viscosity control Used to increase palatability and pourability Examples of viscosity controlling agents are -PVP -cellulose derivatives e. General apperance depends on colour and clarity

4. STABILITY a. Chemical stability Ex- effect of amino acids on stability of aspirin in propylene glycol solutions. b. Physical stability Oral liquids are stable if it retains viscosity, colour, taste and odour throughout its shelf life

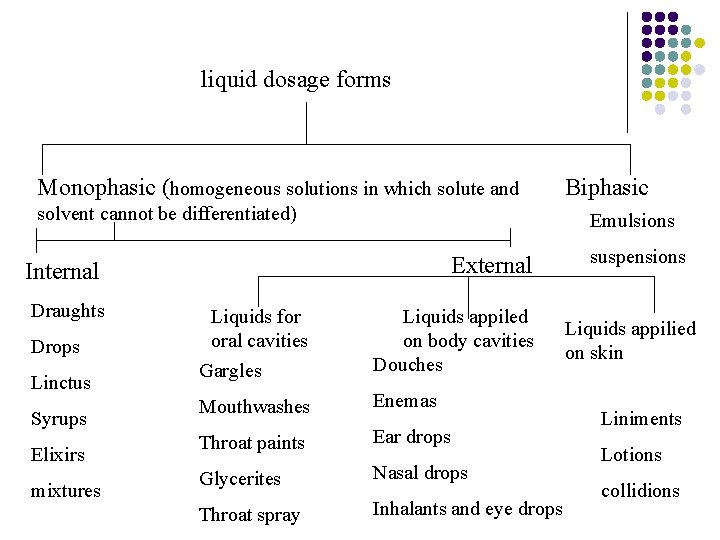
liquid dosage forms Monophasic (homogeneous solutions in which solute and Biphasic solvent cannot be differentiated) Emulsions External Internal Draughts Drops Linctus Syrups Elixirs mixtures Liquids for oral cavities Gargles Liquids appiled on body cavities Douches Mouthwashes Enemas Throat paints Ear drops Glycerites Nasal drops Throat spray Inhalants and eye drops suspensions Liquids appilied on skin Liniments Lotions collidions

ORAL MONOPHASIC DOSAGE FORM Draughts Drops Linctuses Syrups Elixirs Mixtures l

A. Draughts It is simple solution (mixture) which contains a single dose. l Each dose is sent in separate bottle. Total volume is 50 ml. l It is essential to be labelled with storage conditions on draughts. l It is given very early expiry date (48 hours). Examples: 1. Ipecacuanha emetic draughts-BPC (Treatment of poison) 2. Paraldehyde draughts- Oxidized to acetic acid and cause death l Do not use, if the solution is discolored.

Classification of mixtures Mixtures containing soluble substances Eg: Carminative mixtures • Mixtures containing diffusible solids Eg: Bismuth, magnesium carbonate • Mixtures containing in diffusible solids Eg: Salicylic acid, quinine salicylate • Mixtures containing precipitates forming liquid Eg: potassium iodide •

D. SYRUPS: “It is concentrated aqueous solution of sucrose mixed with solution of medicaments and others additives. ” l Generally syrup is prepared by sucrose but is partly replaced by dextrose or other polyhydric alcohols to reduce crystallisation of sucrose or to increase solubility of medicaments and other additives. l Consists of 85% sugars l 2 types of syrups Non medicated syrup Medicated syrup l

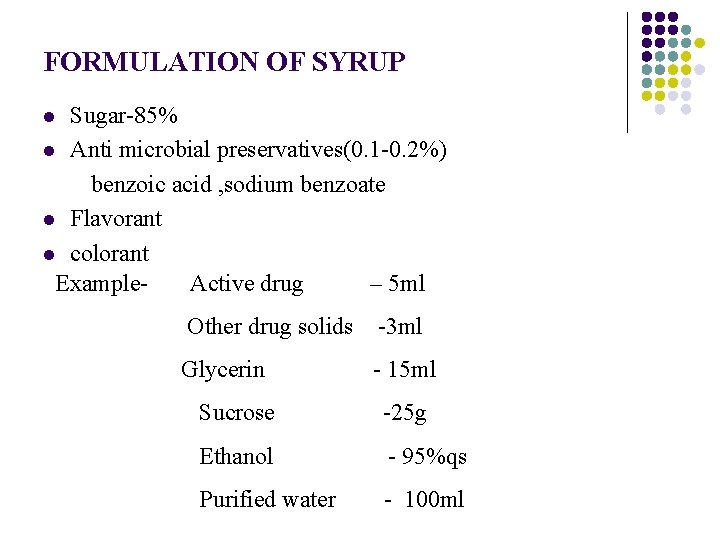
FORMULATION OF SYRUP Sugar-85% l Anti microbial preservatives(0. 1 -0. 2%) benzoic acid , sodium benzoate l Flavorant l colorant Example- Active drug – 5 ml l Other drug solids -3 ml Glycerin - 15 ml Sucrose -25 g Ethanol - 95%qs Purified water - 100 ml

Function of Syrups Sweetening agent l Good antioxidant l Preservatives l Demulcents and l soothing agent l EXAMPLES OF SYRUPS: Codeine phosphate syrup Squill syrup l

Syrups are used formulating Antibiotics Antitussives Antihistamines Vitamins Analgesic/antipyretic PREPARATION OF SYRUP – 1. Solution with heat: For non-volatile, thermostable drugs 2. Agitation without heat: For volatile, thermolabile drugs 3. Addition of medicating liquid to syrup: For liquid medicaments such as extracts, tinctures 4. Percolation:

E. ELIXIRS Elixirs are clear, pleasantly-flavoures, sweetened Hydro alcoholic liquid intended for oral use. 2 types of elixirs • Medicated elixir • Non medicated elixir

ADDITIVES 1. Chemical Stabilizers: p. H Adjustment Sequestering agents- like EDTA 2. Colouring Agents: Amaranth Compound Tartrazine 3. Flavouring Agents: Lemon spirit , Compound orange spirit 4. Sweetening Agents: Invert Syrup, Sodium Sachharin 5. preservatives: 20% Alcohol as vehicle Propylene glycol

Formulation of phenobarbital elixir phenobarbital - 4 g orange oil - 0. 25 ml propylene glycol - 100 ml alcohol - 200 ml sorbitol solution - 600 ml colour - qs purified water - 1000 ml EXAMPLES Chloral Elixir pediatric Paracetamol Elixir Ephedrine Elixir Phenobarbitone Elixir


FORMULATION OF LINCTUSES 1. Vehicle 2. Additives l 1. Vehicles: a. Syrup b. Tolu Syrup l It also provides aromatic odour and flavour, it has also mild expectorant action. l Due to high content (68%) of sucrose, solution tends to crystallise, so stored at constant temperature. l Due to high solid and low water content, dissolution of medicament is slow. Sometime glycerol is used as Auxiliary Vehicle. l

2. Additives A. Chemical Stabilizers : Linctuses are self stabilized due to syrup, so no need of any other stabilizer. B. Colouring Agent: Coal-tar dyes Ex: Compound Tartrazine solution C. Flavouring Agent: Syrup itself act as masking agent. Some fruit flavours used are; Lemon and Black current D. Preservatives: Syrup has preservative action due to high osmotic pressure. Tolu syrup contain Benzoic acid and cinnamic acid. Some other preservatives are -Chloroform spirit -Benzoic acid spirit solution

Liquids for Special Use (Oral Cavity) l l l Gargles Mouth-washes Throat paints Glycerites Throat Spray

A. Gargles: -Gargles are aqueous clear solutions intended to be used in the mouth and throat. . Gargles are pleasantly flavoured and 5 -9. 5 p. H appear to be safe. -Some therapeutic agents used in gargles are antibiotics, antiseptics , local anesthetics, Analgesic, Deodorants and Astringents. Label : “For external use only” Examples: Phenol gargles Potassium chlorate and phenol gargles Thymol glycerin compound

B. Mouth Washes - Mouth washes are aqueous solution used to cleanse and deodorise the buccal cavity. - used for its deodorants, refreshing or antiseptic property. Mouth washes generally contain antibiotic agents or astringent , alcohol, glycerin, sweeteners and surfactants, flavouring and coloring agents. Label : “For external use only” Examples: - Alkaline phenol mouth wash Hydrogen peroxide mouth wash Buffer sodium perborate mouth wash Compound sod. Chloride mouth wash

C. Throat Paints Throat paints are viscous liquid preparations applied to mucous surfaces. l They may be aqueous or alcoholic solution more viscous due to high content of glycerin and prolong the action of medicaments. l Throat paints are used for the mouth and throat infection. l contains – antiseptic, astringent and analgesic. CONTAINERS: Dispensed in airtight colored bottle in order to distinguish from preparation meant for internal use. EXAMPLES: Compound iodine paints Crystal violet paint l

D. Throat Spray solutions are aqueous alcoholic or glycerin solution intended for throat or nose by means of atomizer or nebulizer. l The spray device should produce coarse droplets for upper respiratory tract, while fine droplets for lungs (produced by nebulizer). l Spray solution contain antibiotics, antihistaminic, vasoconstrictors, alcohol and suitable solubilizing and wetting agents. Spray solution contain chlorbutol as an antibacterial and antifungal agent. l EXAMPLES: Adrenaline and Atropine spray compound-BPC. l

LIQUIDS FOR SPECIAL USE (OTHER THAN ORAL CAVITY): l l l DOUCHES ENEMAS EAR-DROPS NASAL DROPS INHALANTS/SPRAY/AEROSOLS Eye drops

A. Douches is medicated aqueous solution for rinsing body cavity applied at lower pressure like l Eye douches l Pharyngeal douches l Nasal douches l Vaginal douches l Bladder douches l Rectum douches l Generally three cavities are widely used ; For vaginal purpose termed as Douches For bladder purpose termed as Irrigation For rectum purpose termed as Enemas. l

-Douches are restricted to vaginal solution have cleansing or antiseptic action. -Promote healing or as an astringent. -Vaginal douches must be sterile if used after child birth or surgery. -Bladder irrigation must always be sterile as bladder is a sterile organ. -Douches dispensed as concentrated forms and directions for dilution with warm water.

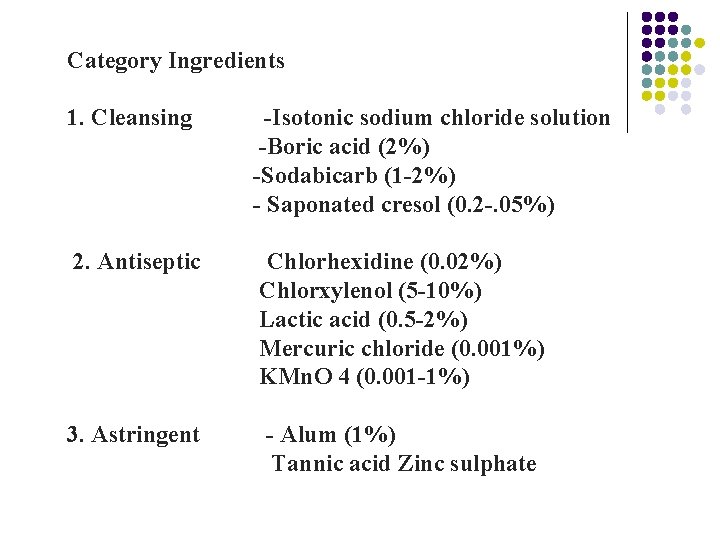
Category Ingredients 1. Cleansing -Isotonic sodium chloride solution -Boric acid (2%) -Sodabicarb (1 -2%) - Saponated cresol (0. 2 -. 05%) 2. Antiseptic Chlorhexidine (0. 02%) Chlorxylenol (5 -10%) Lactic acid (0. 5 -2%) Mercuric chloride (0. 001%) KMn. O 4 (0. 001 -1%) 3. Astringent - Alum (1%) Tannic acid Zinc sulphate

B. ENEMAS “Enemas are aqueous or oily solution or suspension or oil in water emulsion introduced into rectum or colon for cleansing , therapeutic or diagnostic purpose. l Types of Enemas: 1. Cleansing enemas 2. Therapeutic enemas. 3. Diagnostic enemas l

1. CLEANSING ENEMAS: Cleansing enemas used to evacuate feaces in constipation or before an operation. Two types: A. By stimulating of peristalsis i. Large volume: Plain water Soft soap Turpentine enemas ii. Small volume (Osmotic retention) Sodium phosphate enema Magnesium sulphate enema Sorbitol Sodium chloride Sodabicarb B. By lubricating impacted feaces: Olive oil enemas Araches oil Enemas Glycerin enemas

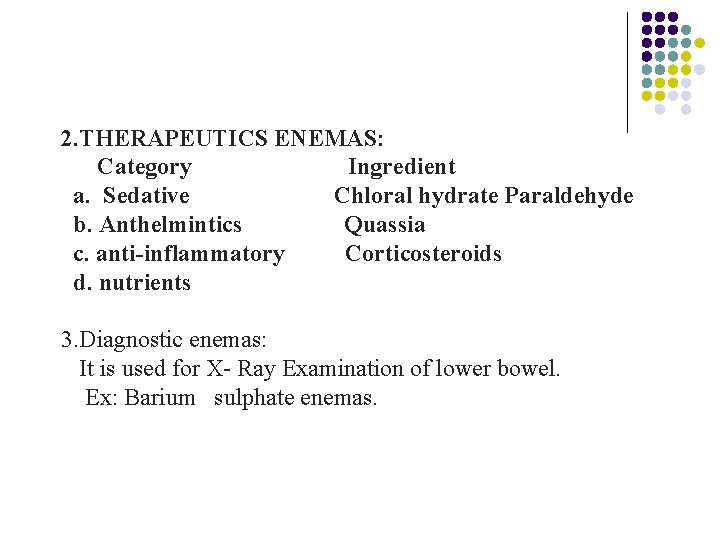
2. THERAPEUTICS ENEMAS: Category Ingredient a. Sedative Chloral hydrate Paraldehyde b. Anthelmintics Quassia c. anti-inflammatory Corticosteroids d. nutrients 3. Diagnostic enemas: It is used for X- Ray Examination of lower bowel. Ex: Barium sulphate enemas.

C. Ear Drops: Ear drops are solutions or suspension of drugs that are used into the ear. VEHICLES: - Water , Propylene glycol, glycerin , PEG-4000 , Dil. Alcohol , Haxylene glycol uses category 1. For mild infection Antibiotics, anti bacterial 2. For softening wax Hydrogen peroxide, sodabicarb 3. For cleansing Spirit 4. Drying weeping surface Astringent, ammonium acetate 5. antiseptic- anaesthesic phenol

CONTAINERS: Coloured fluted glass bottle with dropper LABELLED: - not to be taken orally Store in a cool place Not to be diluted List of official ear drops: Chloramphenicol ear drops Hydrocortisone. Hydrogen peroxide E. D. Phenol E. D. Sodium Bicarbonate E. D.

D. NASAL DROPS “Nasal drops are liquid preparation and may be aqueous or oily for instillation into nostrils. ” The following category can be formulated for nasal drops: - sympathomimetics ------- vasoconstrictor (ephedrine) Anti niflammatory ------ corticosteroids Anti bacterial Anti histamine Local anaesthetics

Nasal Vehicles: they should possess following properties: - p. H range of 5. 5 -7. 5. - Have mild buffer capacity. - Tonicity equivalent to normal saline. - The viscosity should not exceed the normal viscosity of nasal mucus. Contain antimicrobial agents Containers : Fluted color glass bottle with plastic screw cap and dropper. Label: -FOR EXTERNAL USE ONLY - STORE IN A COOL PLACE

E. Nasal inhalation l l l Inhalation are solution or suspension of volatile aromatic medicaments in alcohol, or an alcoholic preparation. And the vapour of which is inhaled to lower respiratory tract. If the ingredient is volatile at room temperature, it may be placed on an absorbent pad and inhaled. If not, poured on hot surface of water (65 C) and vapour is inhaled. Inhalation which requires hot water consists alcoholic solution mixture of medicaments with water and light magnesium carbonate. Containers: White fluted bottle

Label: SHAKE BOTTLE BEFORE USE Examples: Benzoin inhalation Menthol and benzoin inhalation Menthol and eucalyptus inhalation Epinephrine inhalation.

Nasal Sprays Spray solutions are aqueous alcoholic or glycerin solutions in the form of coarse droplets of finely divided solids intended to be applied to the nose or throat by means of atomizer. l The type of atomizer used depends upon the viscosity of the spray solution. l Oily solutions were formerly used, but are no longer used as they retard ciliary action and enter in trachea and cause lipoidal pneumonia. l Examples: Anti inflammatory (corticosteroids) Antihistaminics --Sympathomimetics Local anaesthetics -- Antimigraine l

F. EYE DROPS l l l Sterile, aqueous/oily solutions or suspensions intended for instillation in eye sac. Eye drops may contain buffers, stabilizing agents, dispersing agents, solubilizing agents, anti-oxidants & agents required for tonicity/ viscosity adjustment. Single dose container should not contain anti-microbial preservative. In case of multi dose container a dropper should be supplied with it for administration. Maximum size of such containers is 10 ml.

LIQUIDS FOR EXTERNAL USE l l LINIMENTS LOTIONS

A. LINIMENTS l l l l “Liniments are fluid, semi-fluid or occasionally semisolid preparations intended for external application. ” They may be alcoholic or oily solution or emulsion. Liniments containing substances have following properties. Analgesic Rubefacient Counter Irritants Soothing Stimulating

In case of monophasic (solution) liniments two types of solvents are used. 1. Alcohol - Soap liniment - Aconite liniments. 2. Oil - Camphor Liniments - Methyl Liniments - Arachis and cotton seed oil is used. It is less irritant than alcohol. It is spread more easily. -Liniments may be applied with or without friction. It may be painted or applied on skin by brush. - Liniments should not be applied on broken skin. - Liniments contains volatile ingredients so cold storage is necessary and kept away from flame

PREPARATION: It can be prepared as solution or emulsion as the case may be. CONTAINER: Coloured fluted bottle. LABEL: FOR EXTERNAL USE ONLY NOT FOR BROKEN SKIN STORE IN COOL PLACE INFLAMMABLE

B. LOTIONS l l “Lotion are liquid preparations intended for external application or for special use. It may be aqueous or alcoholic solution or suspension referred as SHAKE LOTION. In aqueous or alcoholic solution, the main ingredients are salts. Alcohol causes defatting, countered by addition of castor oil. Sometimes salt dissolved in water- aqueous solution, termed as EYE-LOTION, meant for eyes.

-On application of shake lotion on skin, the water evaporates, leaving residue of medicaments on skin. - The evaporation causes cooling effect. -Lotions are applied to inflammed area. -The use of alcohol hasten (faster) drying and enhance cooling. - The use of glycerin keeps skin moist and promote adherence of residue powder on skin. - The suspending agent like Na C. M. C. may be used to assist dispersion. -Lotion liable to microbial growth, so includes proper preservatives. Lotion used for local cooling, soothing, protective, drying or moisturizing properties.

Lotion includes- antifungal, anti-inflammatory, anti- infective, anti pyretic and local anesthetic agents. EXAMPLES: Lead lotion - lead subacetate in water Salicylic lotion - salicylic acid in alcohol CONTAINER: Fluted bottle, closed with plastic screw cap. LABEL: FOR EXTERNAL USE ONLY DILUTED LOTION NOT TO BE USED LATED THAN ONE MONTH AFTER ISSUE.

REFERENCES -The theory and practice of industrial pharmacy. leon lachman and lieberman. -Ansel’s pharmaceutical dosage forms and drug delivery systems -Dispensing pharmacy cooper and gunns -Aulton.
