Kinetic Molecular Theory Kinetic Molecular Theory l So

- Slides: 16

Kinetic Molecular Theory

Kinetic Molecular Theory l So far we have considered “what happens, ” but not “why. ” l In science, “what” always comes before “why. ”

Kinetic Molecular Theory Assumptions: 1. Gas particles are in rapid motion, colliding with container walls.

Kinetic Molecular Theory

Kinetic Molecular Theory Assumptions: 2. Gas particles have negligible size compared to the distances between them.

Kinetic Molecular Theory l Animation: Visualizing Molecular Motion (many molecules)

Kinetic Molecular Theory Assumptions: 3. Gas particles have no attraction for one another.

Kinetic Molecular Theory Assumptions: 4. Absolute temperature of the gas is a measure of the average kinetic energy of the gas particles.

Kinetic Molecular Theory l Animation: Kinetic Molecular Theory/Heat-Transfer

Molecular View of The Ideal Gas Law

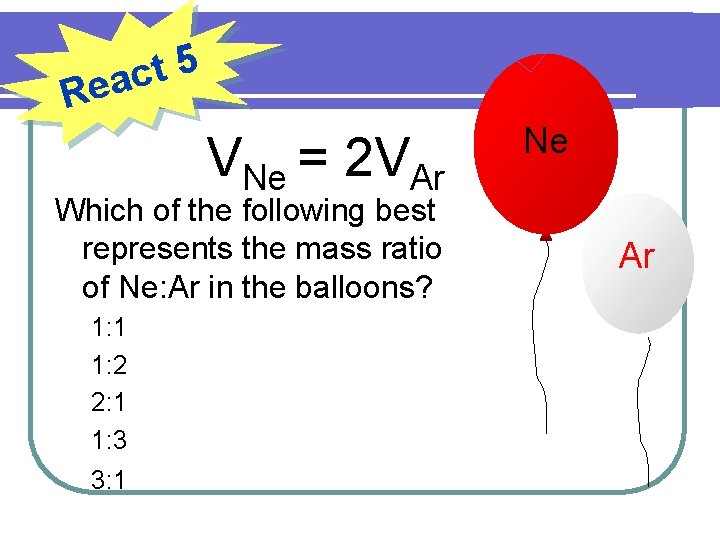
5 t eac R VNe = 2 VAr Which of the following best represents the mass ratio of Ne: Ar in the balloons? 1: 1 1: 2 2: 1 1: 3 3: 1 Ne Ar

Video: Collapsing Can

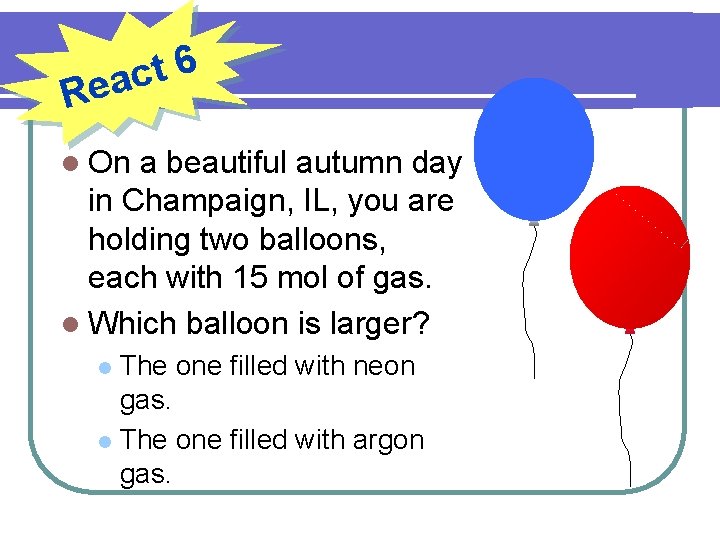
6 t eac R l On a beautiful autumn day in Champaign, IL, you are holding two balloons, each with 15 mol of gas. l Which balloon is larger? The one filled with neon gas. l The one filled with argon gas. l

6 t eac R l On a beautiful autumn day in Champaign, IL, you are holding two balloons, each with 15 mol of gas. l Estimate the volume of each balloon.

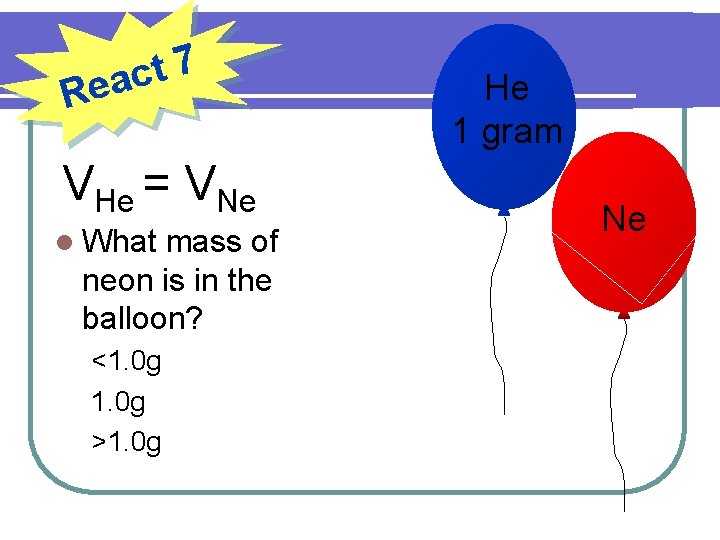
7 t eac R VHe = VNe l What mass of neon is in the balloon? <1. 0 g >1. 0 g He 1 gram Ne

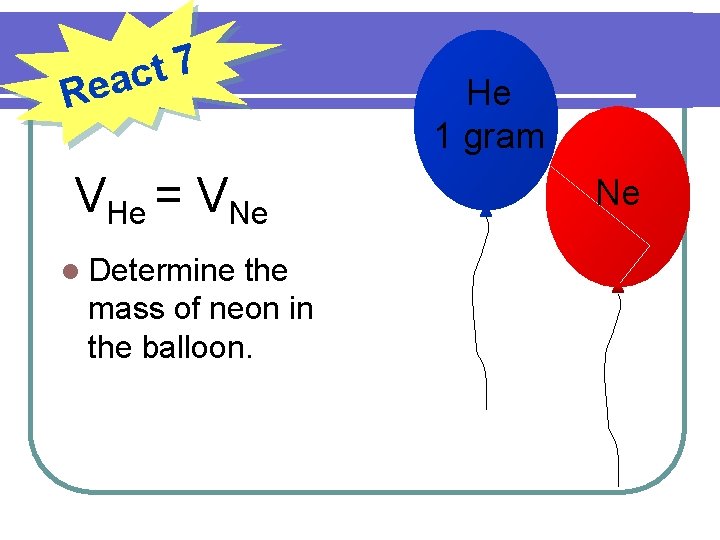
7 t eac R VHe = VNe l Determine the mass of neon in the balloon. He 1 gram Ne
 Kinetic molecular theory of solids
Kinetic molecular theory of solids Kinetic molecular model of gases
Kinetic molecular model of gases Kinetic molecular theory volume
Kinetic molecular theory volume Adhesive force
Adhesive force Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Kinetic energy molecular theory
Kinetic energy molecular theory Kinetic molecular theory def
Kinetic molecular theory def Timeline of kinetic molecular theory
Timeline of kinetic molecular theory Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Basic postulates of kinetic theory of gases
Basic postulates of kinetic theory of gases Kenitic molecular theory
Kenitic molecular theory Postulates of kinetic theory of gases
Postulates of kinetic theory of gases Pv=1/3nmc^2
Pv=1/3nmc^2 Kinetic molecular theory
Kinetic molecular theory Tenets of kinetic molecular theory
Tenets of kinetic molecular theory Covalently bonded substances
Covalently bonded substances Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure































