2 2 Kinetic Theory Kinetic Theory Kinetic Theory








- Slides: 19

2. 2 – Kinetic Theory

Kinetic Theory • Kinetic Theory Demo – Food coloring

• 1738 – Daneil Bernoulli – Swiss mathematician and physicist - published Hydrodynamica – Gases consist of molecules moving rapidly in all directions – These particles is why we feel pressure in fluids – What we perceive as heat is simply the movement(kinetic energy) of the particles


Kinetic Theory • Kinetic Theory explains how particles behave within matter. • Basic Assumptions – All matter is composed of small particles (atoms, molecules, and ions) – Particles are constant and random. – Particles collide with each other transferring kinetic energy.



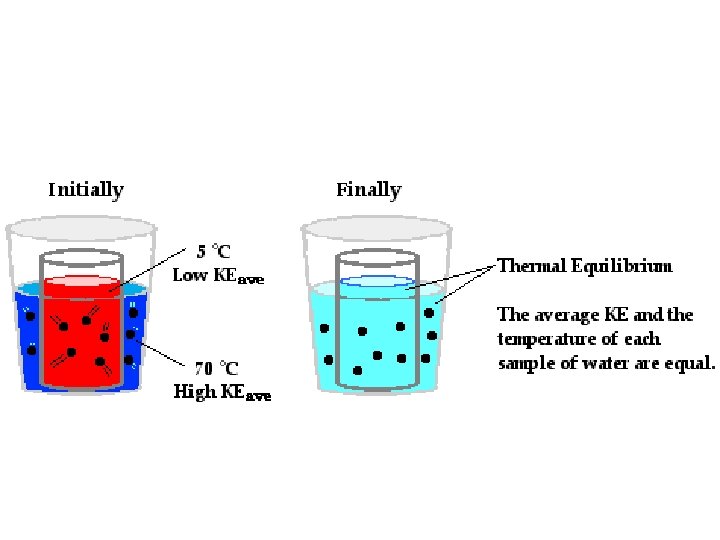
Thermal Energy • Thermal energy is the total energy of a materials particles. • The energy in these particles causes them to move and we measure the average kinetic energy of the particles as temperature. • Temperature – the average kinetic energy of the particles of a material – Molecules of water at 0 ˚C have less energy and less movement that molecules of water at 100 ˚C. – What is it called when particles in a substance stop moving completely?

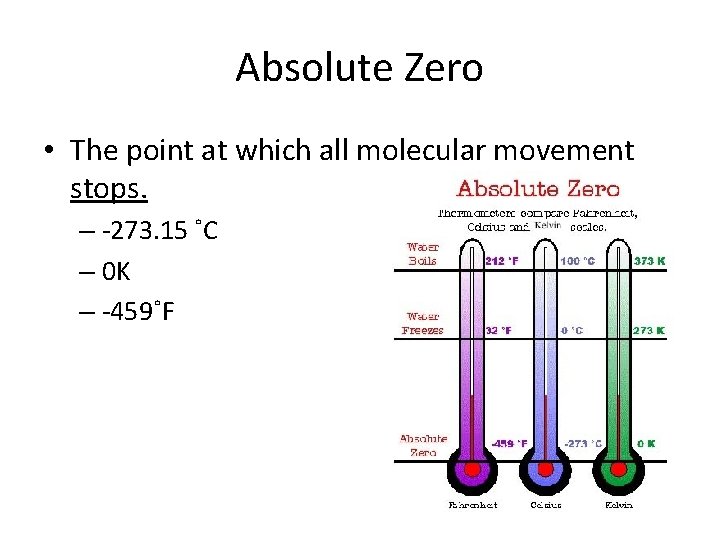
Absolute Zero • The point at which all molecular movement stops. – -273. 15 ˚C – 0 K – -459˚F


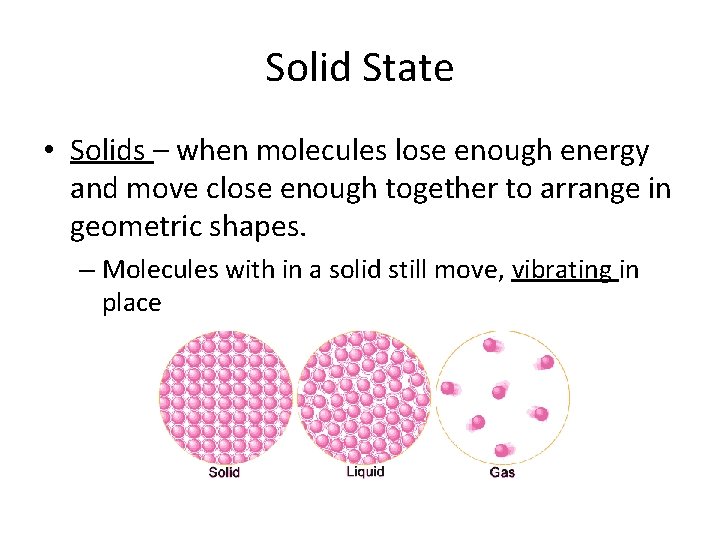
Solid State • Solids – when molecules lose enough energy and move close enough together to arrange in geometric shapes. – Molecules with in a solid still move, vibrating in place

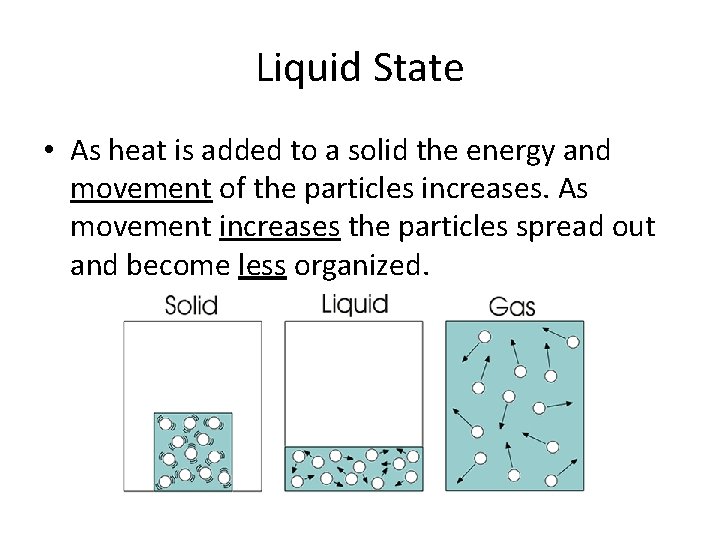
Liquid State • As heat is added to a solid the energy and movement of the particles increases. As movement increases the particles spread out and become less organized.

Gas State • Gas particles have the ability to completely overcome the attractions between them. – Gases do not have a fixed volume or shape

In summary….

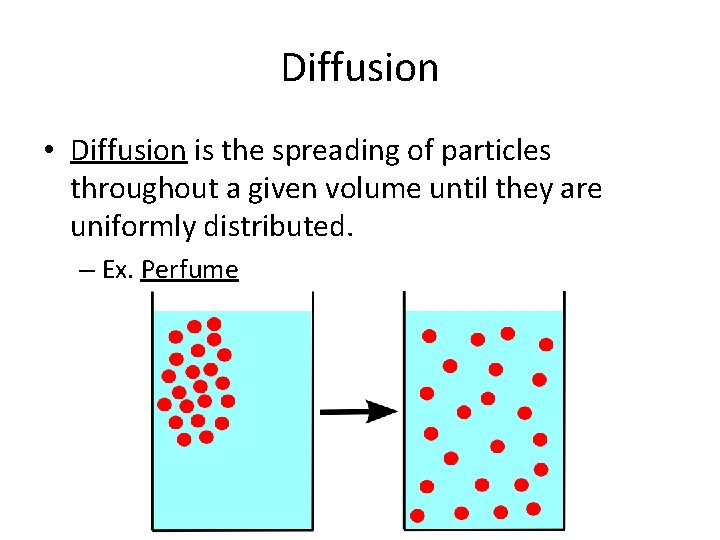
Diffusion • Diffusion is the spreading of particles throughout a given volume until they are uniformly distributed. – Ex. Perfume

Other Phases of Matter • Plasma – matter that is composed of postively and negatively charged particles. This is called by the seperation of electrons from nuclei at very high temperatures. – Ex. Lightning Bolts, Neon and Fluorescent tubes, auroras • Bose-Einstein Condensates • Non-Newtonian Fluids

• Other States of Matter

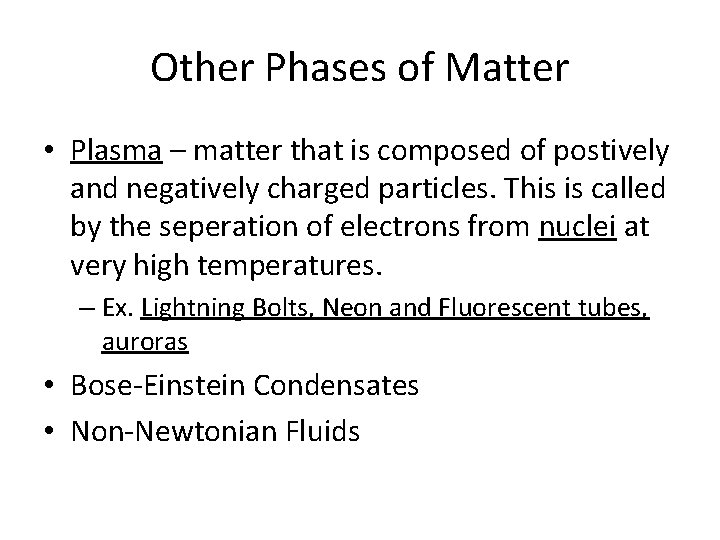
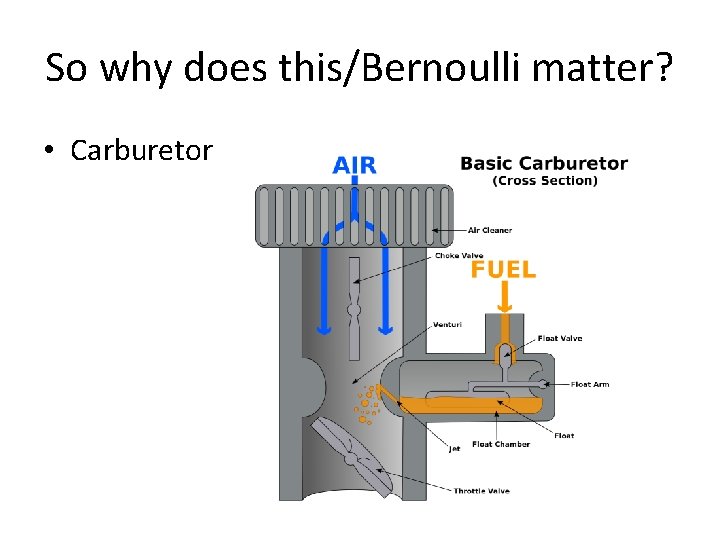
So why does this/Bernoulli matter? • Carburetor

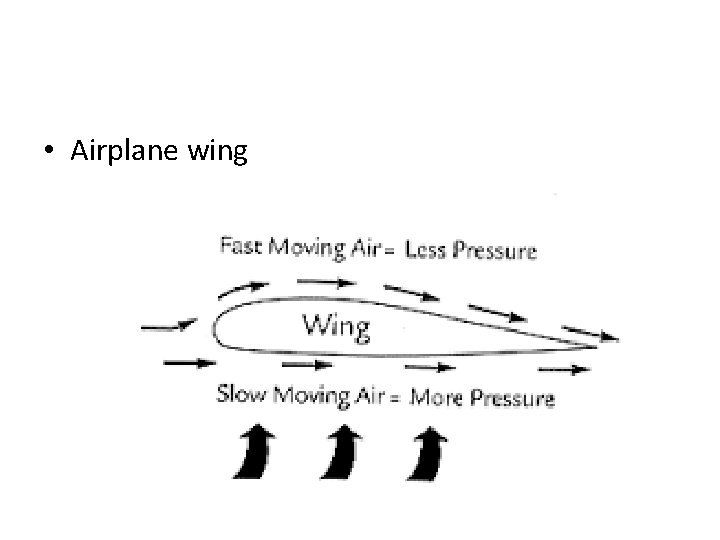
• Airplane wing