Solids Liquids and Gases Kinetic Theory The kinetic






- Slides: 21

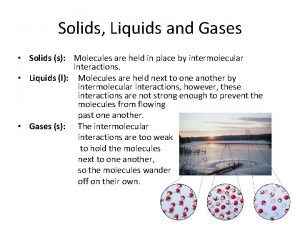
Solids, Liquids, and Gases

Kinetic Theory • The kinetic theory is an explanation of how particles in matter behave. • The three assumptions of the kinetic theory are as follows: – All matter is composed of small particles (atoms, molecules, and ions). – These particles are in constant, random motion. – These particles are colliding with each other and the walls of their container.

Kinetic Theory (2) • These particles do lose some energy during collisions with other particles. • But the amount of energy lost is very small and can be neglected in most cases. • At higher temperature, particles move faster and bump into one another with more force. • At lower temperatures, they move with less energy.

States of Matter • Basis of classification of the four states of matter: – Based upon particle arrangement. – Based upon energy of particles. – Based upon distance between particles. – On Earth, matter is usually found as solids, liquids, or gases. – Plasma is the most common state of matter in the universe, but it is rarely found on Earth.

States of Matter (2) • An everyday activity such as eating lunch may include solids, liquids, and gases. • Can you identify the states of matter present in the photo shown?

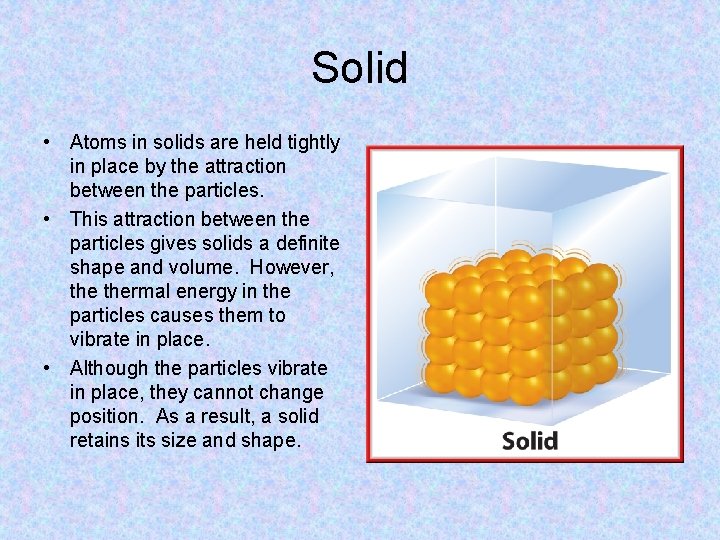
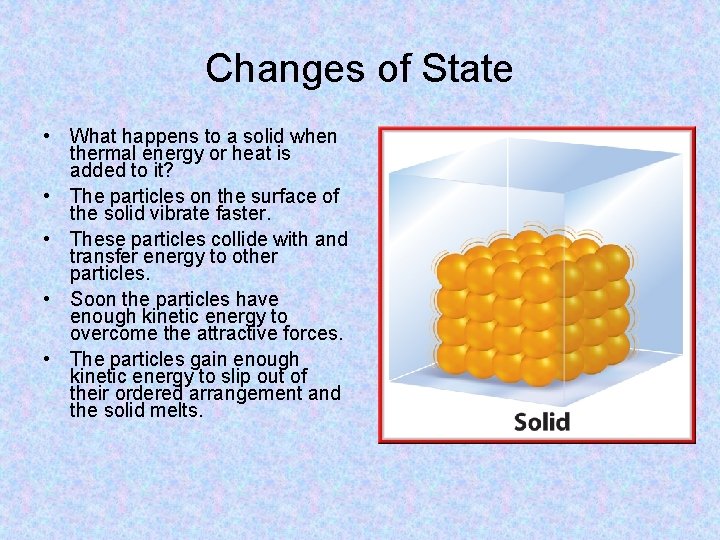
Solid • Atoms in solids are held tightly in place by the attraction between the particles. • This attraction between the particles gives solids a definite shape and volume. However, thermal energy in the particles causes them to vibrate in place. • Although the particles vibrate in place, they cannot change position. As a result, a solid retains its size and shape.

Solids (2) Particle Movement Examples

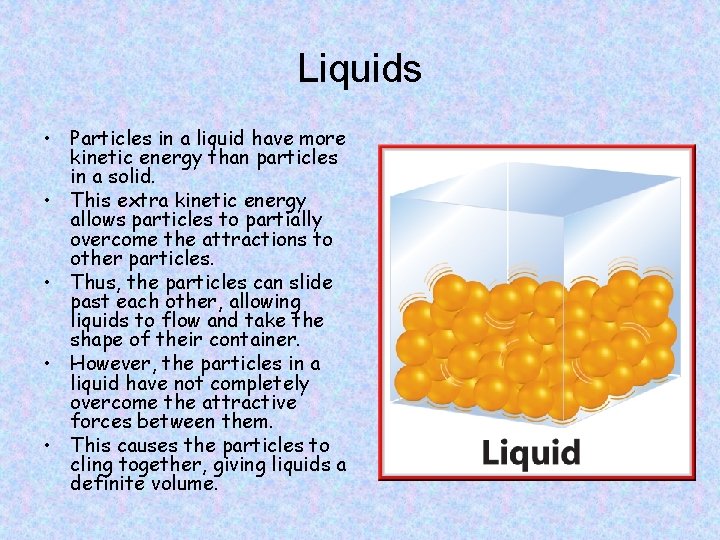
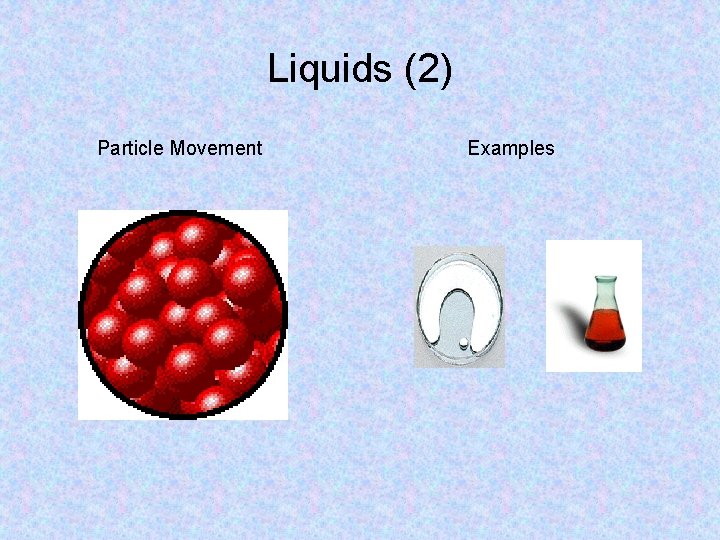
Liquids • Particles in a liquid have more kinetic energy than particles in a solid. • This extra kinetic energy allows particles to partially overcome the attractions to other particles. • Thus, the particles can slide past each other, allowing liquids to flow and take the shape of their container. • However, the particles in a liquid have not completely overcome the attractive forces between them. • This causes the particles to cling together, giving liquids a definite volume.

Liquids (2) Particle Movement Examples

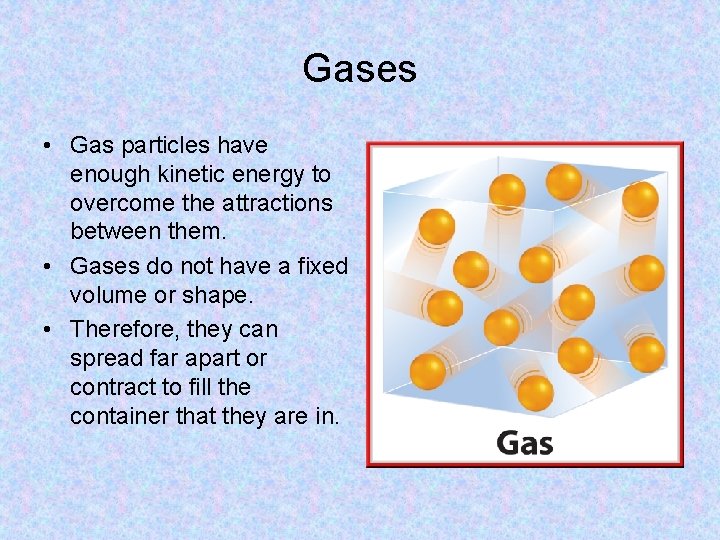
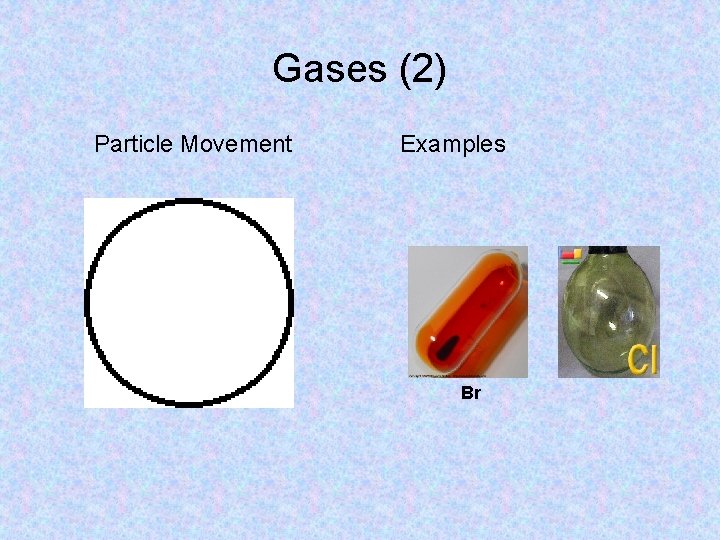
Gases • Gas particles have enough kinetic energy to overcome the attractions between them. • Gases do not have a fixed volume or shape. • Therefore, they can spread far apart or contract to fill the container that they are in.

Gases (2) Particle Movement Examples Br

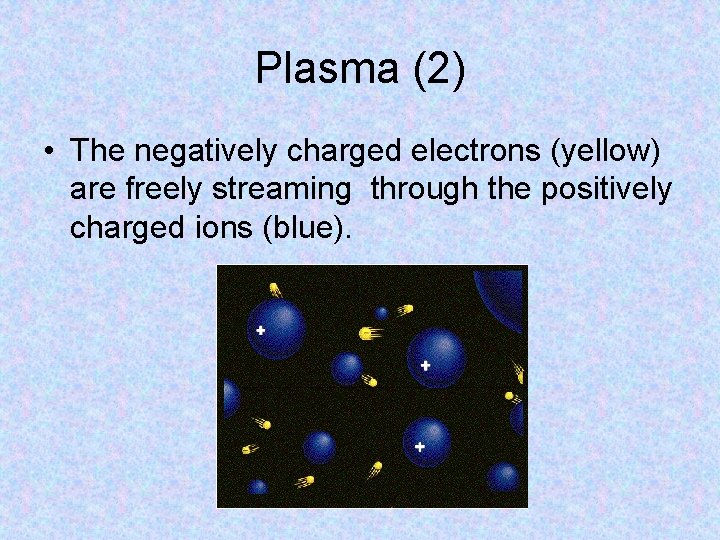
Plasma • Plasma is matter consisting of positively and negatively charged particles. • Matter enters the plasma state when it is heated to such a high temperature that some of the atoms begin to break apart. • A type of plasma is used on Earth to make neon and fluorescent lights. Instead of heating the gases to an extremely high temperature, an electrical current is passed through them. The current strips the electrons from the atoms, producing plasma.

Plasma (2) • The negatively charged electrons (yellow) are freely streaming through the positively charged ions (blue).

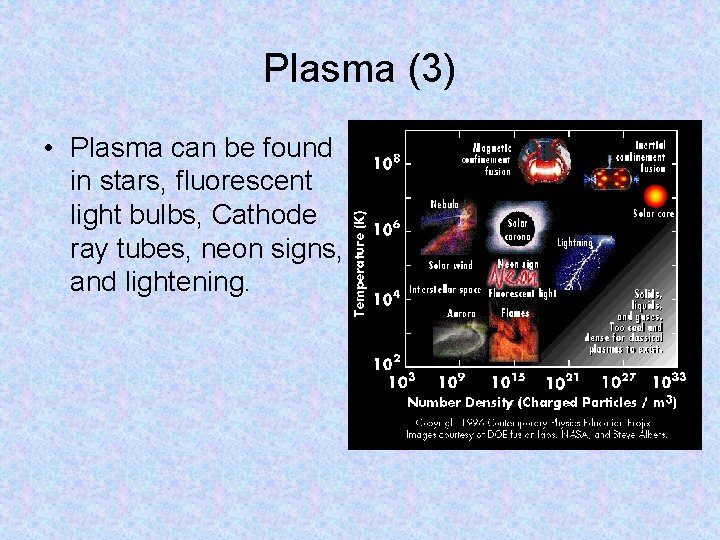
Plasma (3) • Plasma can be found in stars, fluorescent light bulbs, Cathode ray tubes, neon signs, and lightening.

Changes of State • What happens to a solid when thermal energy or heat is added to it? • The particles on the surface of the solid vibrate faster. • These particles collide with and transfer energy to other particles. • Soon the particles have enough kinetic energy to overcome the attractive forces. • The particles gain enough kinetic energy to slip out of their ordered arrangement and the solid melts.

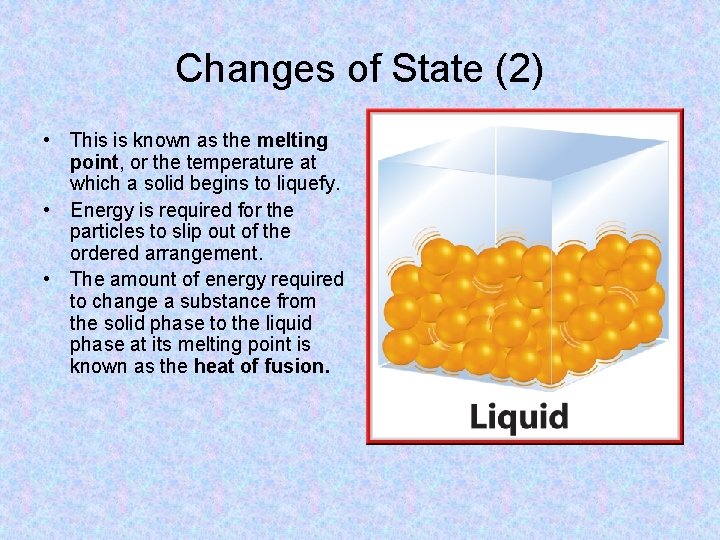
Changes of State (2) • This is known as the melting point, or the temperature at which a solid begins to liquefy. • Energy is required for the particles to slip out of the ordered arrangement. • The amount of energy required to change a substance from the solid phase to the liquid phase at its melting point is known as the heat of fusion.

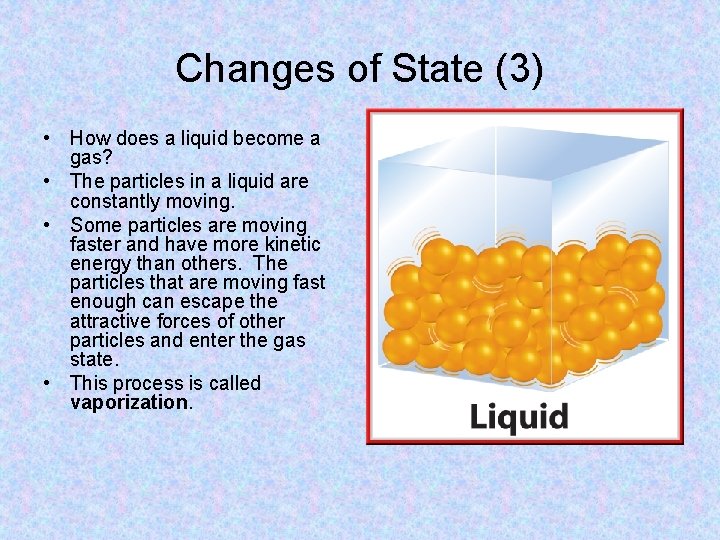
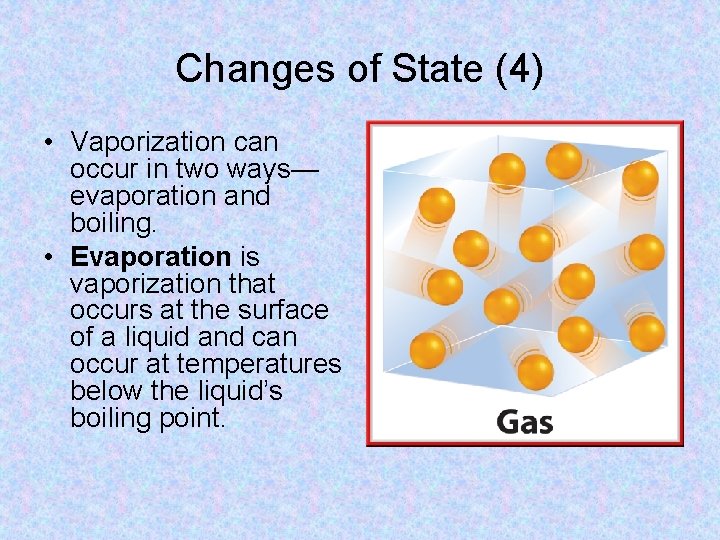
Changes of State (3) • How does a liquid become a gas? • The particles in a liquid are constantly moving. • Some particles are moving faster and have more kinetic energy than others. The particles that are moving fast enough can escape the attractive forces of other particles and enter the gas state. • This process is called vaporization.

Changes of State (4) • Vaporization can occur in two ways— evaporation and boiling. • Evaporation is vaporization that occurs at the surface of a liquid and can occur at temperatures below the liquid’s boiling point.

Changes of State (5) • The boiling point of a liquid is the temperature at which the pressure of the vapor in the liquid is equal to the external pressure acting on the surface of the liquid. • Heat of vaporization is the amount of energy required for the liquid at its boiling point to become a gas.

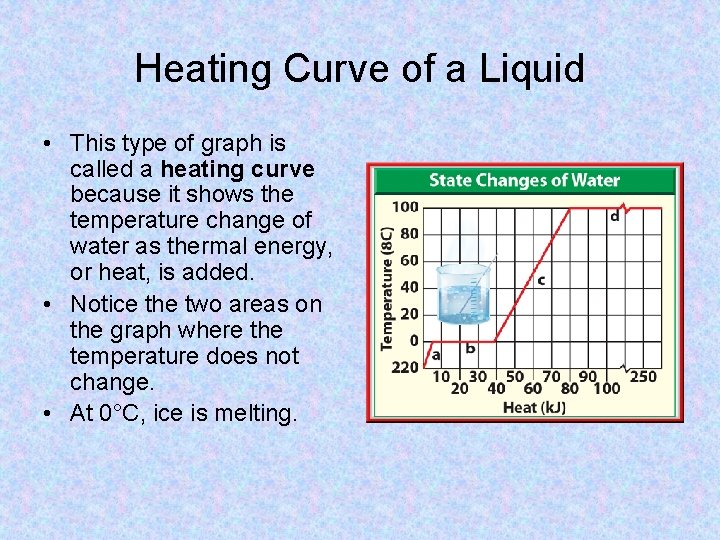
Heating Curve of a Liquid • This type of graph is called a heating curve because it shows the temperature change of water as thermal energy, or heat, is added. • Notice the two areas on the graph where the temperature does not change. • At 0°C, ice is melting.

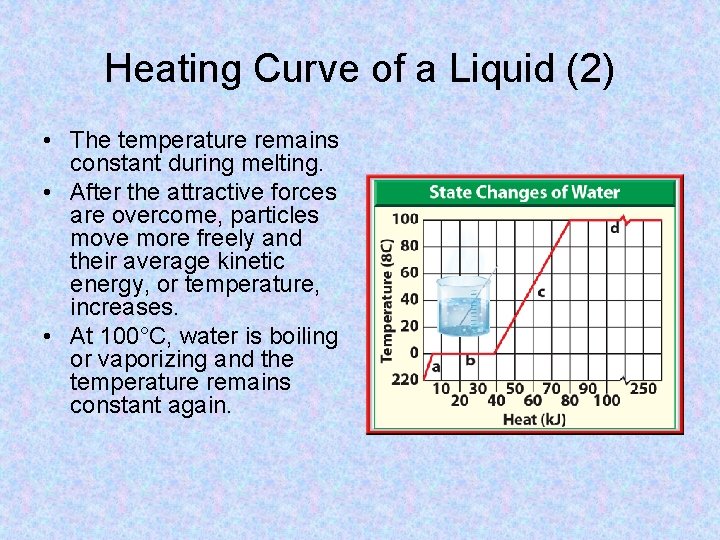
Heating Curve of a Liquid (2) • The temperature remains constant during melting. • After the attractive forces are overcome, particles move more freely and their average kinetic energy, or temperature, increases. • At 100°C, water is boiling or vaporizing and the temperature remains constant again.
 Kinetic molecular theory of solids
Kinetic molecular theory of solids Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Expansion of solids liquids and gases examples
Expansion of solids liquids and gases examples Solids liquids and gases section 2 properties of fluids
Solids liquids and gases section 2 properties of fluids Venn diagram of solid and liquid
Venn diagram of solid and liquid Properties of a solid
Properties of a solid Solid liquid gas examples
Solid liquid gas examples Lesson 1 thermal energy and the behavior of matter
Lesson 1 thermal energy and the behavior of matter Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Particle movement in solids liquids and gases
Particle movement in solids liquids and gases How does sound travel through solids liquids and gases
How does sound travel through solids liquids and gases Matter and its composition
Matter and its composition Motion of particles in solids, liquids and gases
Motion of particles in solids, liquids and gases Why are gases easier to compress than solids or liquids?
Why are gases easier to compress than solids or liquids? Molecular theory of gases and liquids
Molecular theory of gases and liquids Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Solid liquid and gas particles
Solid liquid and gas particles Liquids and solids menu
Liquids and solids menu Liquids and solids
Liquids and solids Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids Kinetic theory of solids
Kinetic theory of solids Constant rate filtration example
Constant rate filtration example