Solids Liquids and Gases Kinetic Theory The kinetic















- Slides: 24

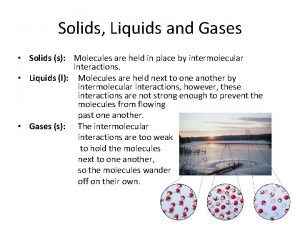
Solids, Liquids, and Gases

Kinetic Theory • The kinetic theory is an explanation of how particles in matter behave. – All matter is composed of small particles (atoms, molecules, ions) – These particles are in constant, random motion. – These particles are colliding with each other and the walls of their container.

Cool Science • http: //youtu. be/b. Ph 1 KXA 8 eg. U

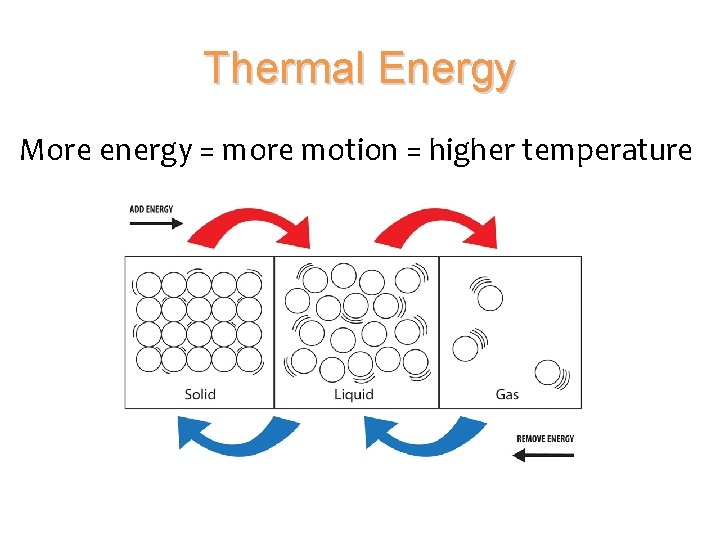
Thermal Energy • All objects have energy • Potential Energy – from the bonds that hold the substance together • Kinetic Energy – from the motion or vibration of the atoms and molecules • The total energy of a material’s particles is called thermal energy.

Thermal Energy More energy = more motion = higher temperature

Temperature • Temperature measures the average kinetic energy of the particles in a substance.

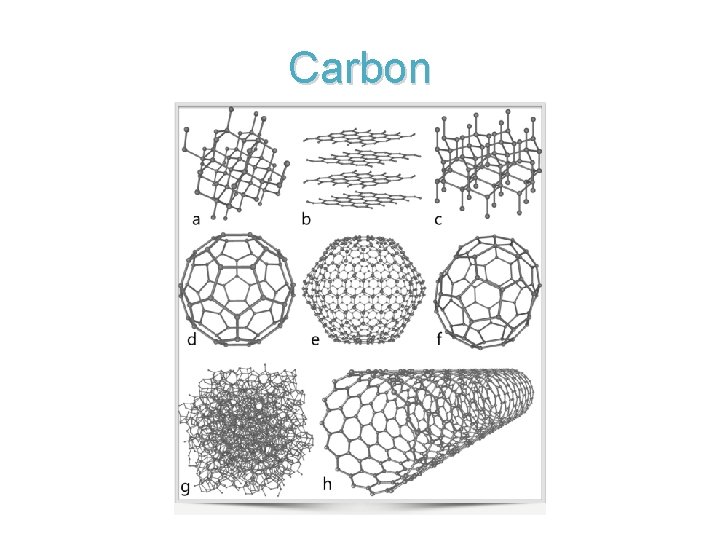
Solids • Particles are closely packed together • Particles have different geometric arrangements. – These arrangements determine the physical and chemical properties.

Carbon

Liquids • Particles have enough energy to break free from the geometric arrangement. • Substance flows and takes the shape of its container. • Melting point – temperature at which a substance goes from solid to liquid • Heat of Fusion – energy required to reach the melting point

Gases • Particles have enough energy to escape the attractive force of the other molecules. • Expand to take the size and shape of their container • Heat of Vaporization – amount of energy required for a liquid to become a gas • Diffusion – spreading out of gas molecules to fill their container.

Boiling • Boiling point – temperature at which a substance goes from a liquid to a gas. • Liquids stay in their container because of air pressure. • Liquids boil because the internal pressure of the liquid becomes equal to the pressure of the air and the molecules are allowed to escape.

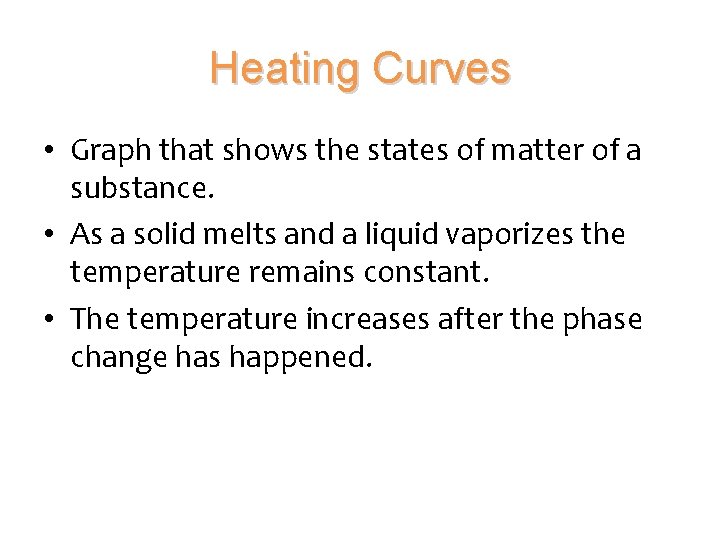
Heating Curves • Graph that shows the states of matter of a substance. • As a solid melts and a liquid vaporizes the temperature remains constant. • The temperature increases after the phase change has happened.

Heating Curves

Plasmas • High temperature gas with positively and negatively charged particles. • Found in lightning and neon signs

Thermal Expansion • Increase in the size of a substance when it is heated. • Occurs in most solids, liquids, and gases. • Water is the exception.

Weird Things • Some substances do not react as expected when changing states. – Amorphous Solids • Lack the geometric pattern of other solids • Glass and Plastic • No definite melting/boiling point – Liquid Crystals • Keep their geometric arrangement when liquid • Liquid Crystal Displays – Non-Newtonian Substances • Acts as different states based on certain conditions

Buoyancy • The ability of a fluid (liquid or gas) to exert an upward force on an object immersed in it. • If the buoyant force is equal to the weight of the object, the object will float. • If the buoyant force is less than the weight of the object, the object will sink.

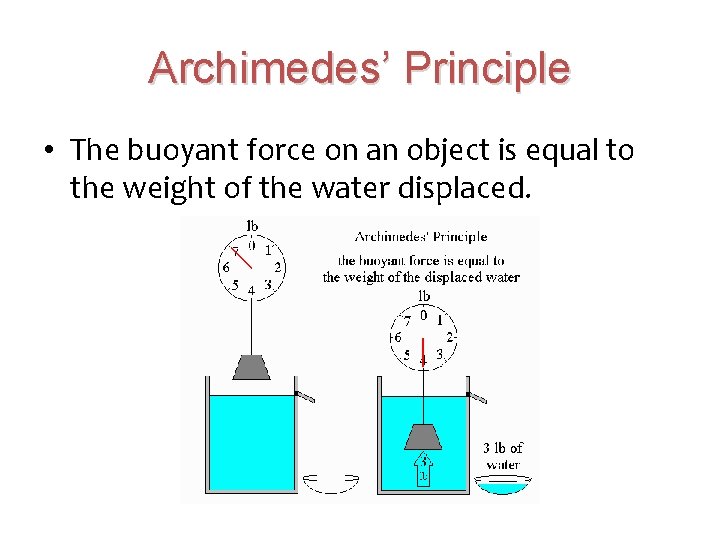
Archimedes’ Principle • The buoyant force on an object is equal to the weight of the water displaced.

Density • • Mass ÷ Volume More dense objects sink Less dense objects float To increase the density you can: – Decrease the volume – Increase the mass • To decrease the density you can: – Increase the volume – Decrease the mass

Pressure • Pressure = Force / Area • To increase the pressure: – Increase the force – Decrease the area • To decrease the pressure: – Decrease the force – Increase the area

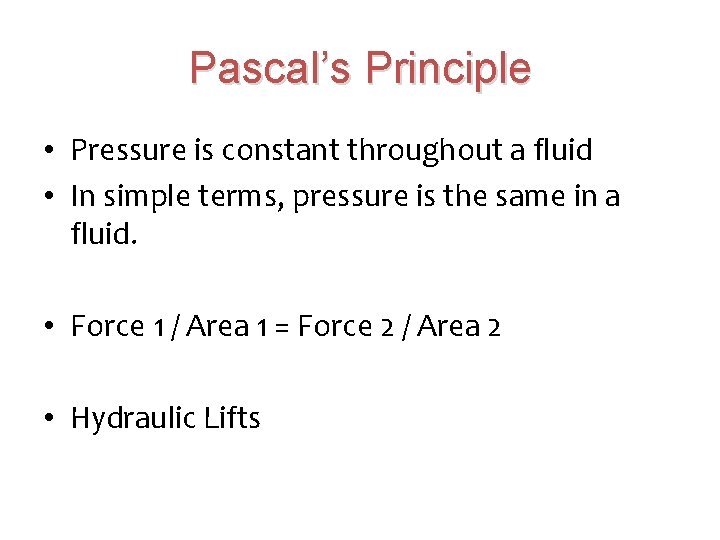
Pascal’s Principle • Pressure is constant throughout a fluid • In simple terms, pressure is the same in a fluid. • Force 1 / Area 1 = Force 2 / Area 2 • Hydraulic Lifts

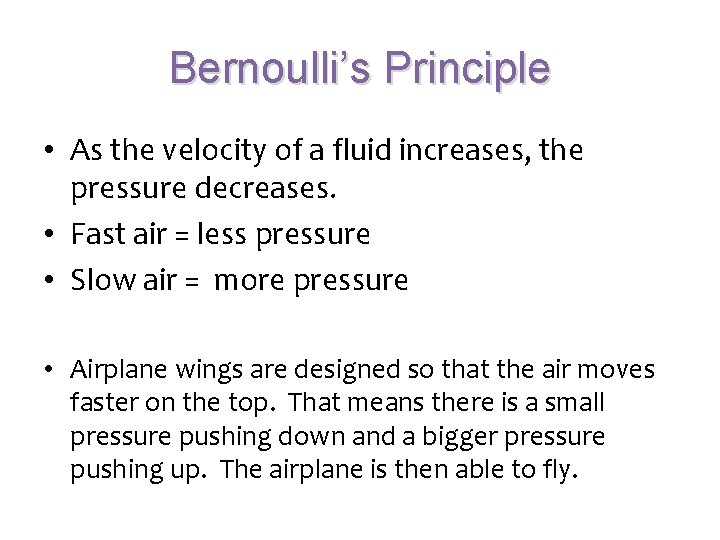
Bernoulli’s Principle • As the velocity of a fluid increases, the pressure decreases. • Fast air = less pressure • Slow air = more pressure • Airplane wings are designed so that the air moves faster on the top. That means there is a small pressure pushing down and a bigger pressure pushing up. The airplane is then able to fly.

Viscosity • The resistance of a fluid to flow • Low viscosity = flows easily (water, milk) • High viscosity = does not flow easily (syrup, ketchup)

Assignment • Section 2 Reinforcement • On the back, answer these questions: – What are two opposing forces that act on an object floating in water? – Why do helium balloons float and non helium balloons sink? – Why are roofs lifted off houses during tornados? – How is it possible for a heavy boat to float on water?
 Chapter 14 solids liquids and gases
Chapter 14 solids liquids and gases Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Expansion of solids liquids and gases examples
Expansion of solids liquids and gases examples Solids liquids and gases section 2 properties of fluids
Solids liquids and gases section 2 properties of fluids Venn diagram of solid and liquid
Venn diagram of solid and liquid What are the properties of solids
What are the properties of solids Solid liquid gas examples
Solid liquid gas examples The science duo physical and chemical changes
The science duo physical and chemical changes Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Particle movement in solids liquids and gases
Particle movement in solids liquids and gases How does sound travel through solids liquids and gases
How does sound travel through solids liquids and gases Properties of solid liquid and gas
Properties of solid liquid and gas Motion of particles in solids, liquids and gases
Motion of particles in solids, liquids and gases Why are gases easy to compress?
Why are gases easy to compress? Molecular theory of gases and liquids
Molecular theory of gases and liquids Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Gas to solid
Gas to solid Liquids and solids menu
Liquids and solids menu Liquids and solids
Liquids and solids Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids The attraction between particles gives solids a definite
The attraction between particles gives solids a definite Specific cake resistance formula
Specific cake resistance formula Kinetic theory for ideal gases
Kinetic theory for ideal gases Kinetic particle theory o level questions
Kinetic particle theory o level questions Kinetic theory of gases
Kinetic theory of gases