Kinetic Molecular Theory KINETIC MOLECULAR THEORY Theory used






- Slides: 11

Kinetic Molecular Theory

KINETIC MOLECULAR THEORY Theory used to describe how all matter is composed. 1. 2. 3. 4. 5. 6. 7. 8. Everything is made of small particles. Particles are always in constant motion. There are empty spaces between the particles. The particles and spaces are so small that they cannot be seen. Solids - particles move slowly (vibrating back and forth) and are held tightly together. Liquids - particles move faster and have small spaces between them. Gases – the particles are far apart and move very quickly. Heat and other forms of energy can excite the particles.

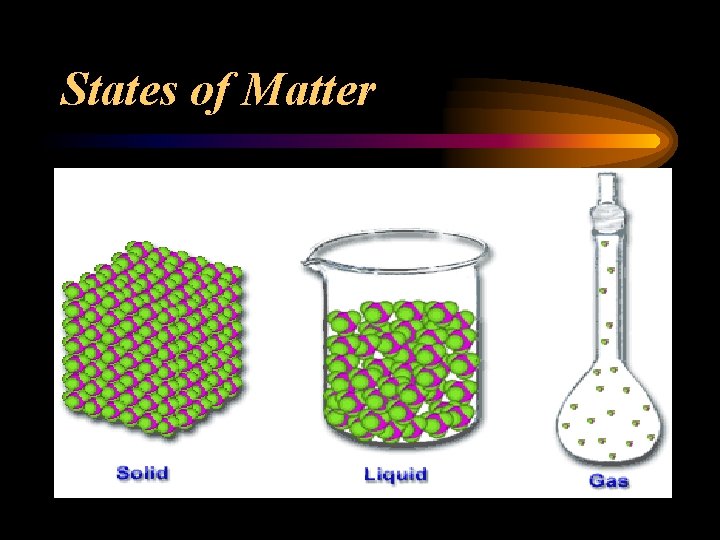
States of Matter

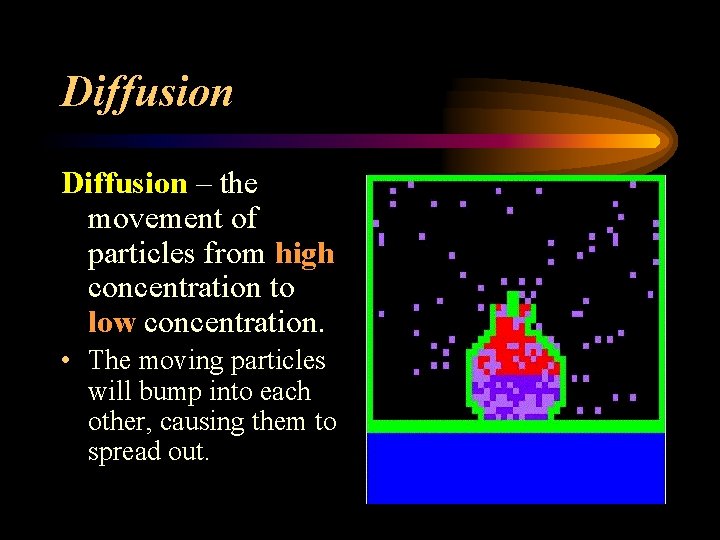
DIFFUSION • What happens when you open a bottle of perfume? • The odour gradually spreads out and eventually fades. • This is evidence of the KMT because the particles are spreading out. • The moving particles will bump into each other, causing them to spread out.

Diffusion – the movement of particles from high concentration to low concentration. • The moving particles will bump into each other, causing them to spread out.

With this in mind … Q: Which phase and temperature will diffuse fastest? Why? A: Gas and hot – particles move fastest in gases and hot temperatures make the particles spread out faster. Q: What is it about solids that allows them to hold their shape? A: Particles do not move fast (just vibrate) and are held close together.

Q: Why do liquids take the shape of their container? A: Liquids spread out – particles can move freely, but are still held together. Q: Why do gases evenly fill whatever container they occupy? A: Particles move fast they spread out to fill the whole container.

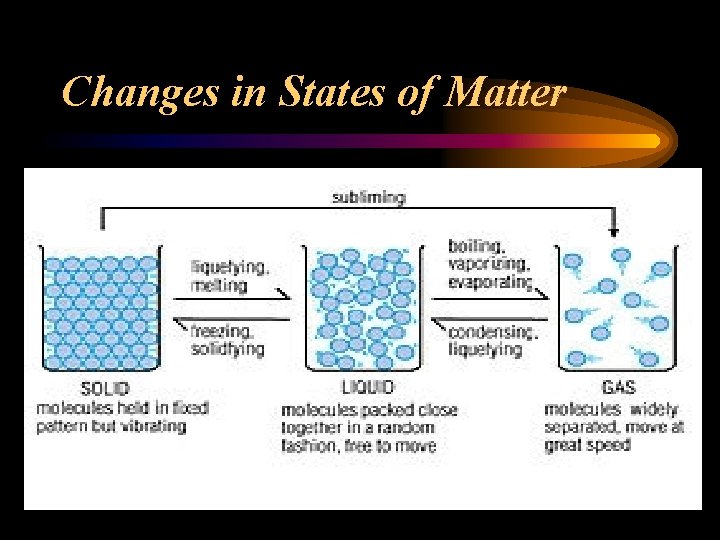
Changes in States of Matter

Melting/Freezing • Melting is the change of state from solid to liquid (the solid has been heated). • Particles vibrate faster and faster until they have enough energy to break away from their fixed positions. • Freezing is the reverse process to melting. As particles cool they loose energy and settle into a fixed position.

Sublimation • Some solids can change directly to a gas without first becoming a liquid • Individual particles can gain enough energy to break completely away from the solid. • The reverse of this process is also called sublimation. • Ex. Ice forming on your windshield at night.

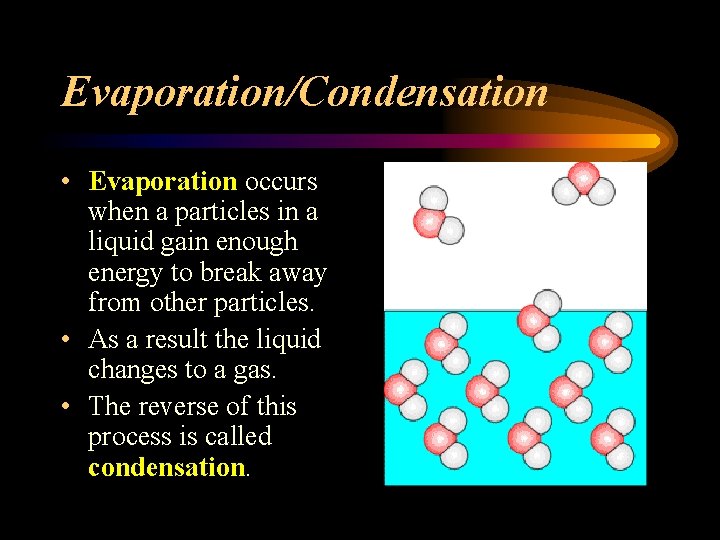
Evaporation/Condensation • Evaporation occurs when a particles in a liquid gain enough energy to break away from other particles. • As a result the liquid changes to a gas. • The reverse of this process is called condensation.
 Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Kinetic theory for ideal gases
Kinetic theory for ideal gases The kinetic molecular theory
The kinetic molecular theory Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Kinetic molecular theory
Kinetic molecular theory Kinetic theory def
Kinetic theory def Theory vs hypothesis
Theory vs hypothesis Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Postulates of kinetic theory of gases
Postulates of kinetic theory of gases Kinetic molecular theory
Kinetic molecular theory Write postulates of kinetic theory of gases
Write postulates of kinetic theory of gases





















