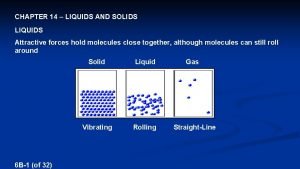
Liquids and Solids Chapter 10 Properties of Liquids





















- Slides: 34

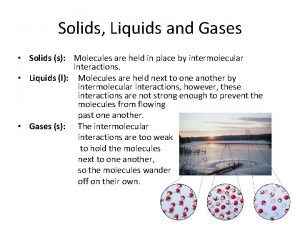
Liquids and Solids Chapter 10

Properties of Liquids and the KMT Liquid- can be described as a form of matter that had definite volume and takes the shape of the bottom of its container Fluid- A substance that can flow and therefore takes the shape of its container

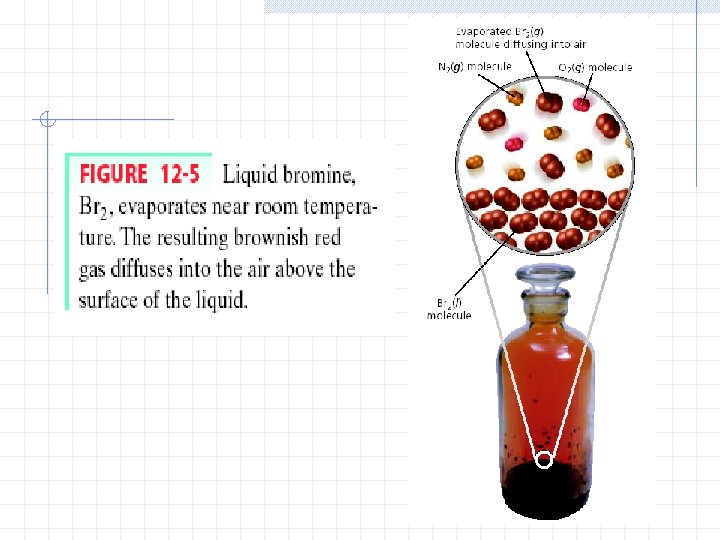
6 properties of liquids 1. Relatively high density- this is a result of the close arrangement of particles 2. Relative incompressibility- liquids are much less compressible than gases because the particles are more closely packed together 3. Ability to diffuse- occurs because of the constant random motion of particles

4. Surface tension- a force that tends to pull adjacent parts of a liquid’s surface together 5. Capillary Action- the attraction of the surface of a liquid to the surface of a solid (root pressure in plants) 6. Evaporation/Vaporizationparticles escape and enter the gas phase


Introduction to Solids When a liquid is cooled, the average energy of its particles decreases The physical change of a liquid to a solid by removal of heat is called freezing (solidification) All liquids freeze…but not necessarily according to what you think (water)

Formation of Solids 1. 2. 3. 4. Properties of solids and the KMT Definite shape and volumeparticles are packed closely together compared to liquids and gases. Definite melting point- the temperature at which a solid becomes a liquid High density and low compressibility Low rate of diffusion

2 Types of Solids 1. Crystalline solids- consists of crystals in which the particles are arranged in an orderly, geometric, repeating pattern 2. Amorphous solids -amorphous means “without shape” -fiberglass, optical fibers, some

Concept Questions 1. How does the kinetic molecular theory explain the following properties of liquids? n A. High density B. ability to diffuse 2. Explain why liquids in a test tube form a meniscus. 3. What is the difference between an amorphous solid and a crystalline solid?

Changes of State Section 12. 3

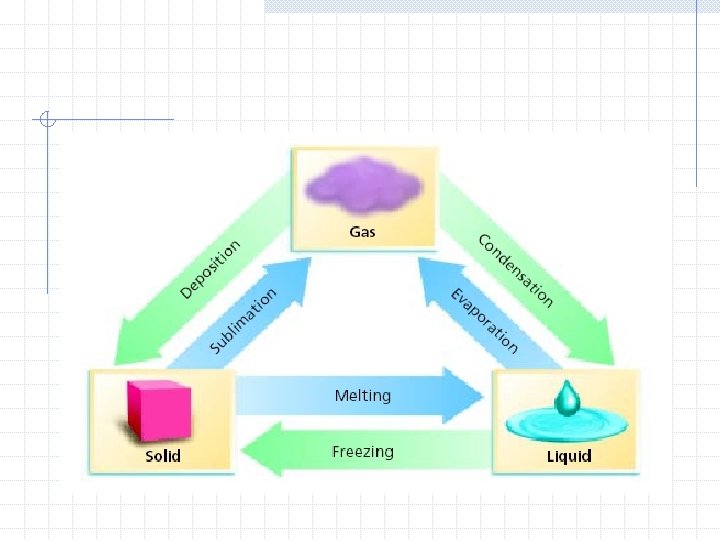
6 phase changes Solid liquid Solid gas Liquid solid Liquid gas Gas liquid Gas solid melting sublimation freezing vaporization condensation deposition


Equilibrium- a dynamic condition in which 2 opposing changes occur at equal rates in a closed system Phase- any part of a system that has uniform composition and properties

An equilibrium equation Liquid + heat energy vapor n Vaporization/evaporation Vapor liquid + heat energy n Condensation Liquid + heat energy vapor n Liquid-vapor equilibrium

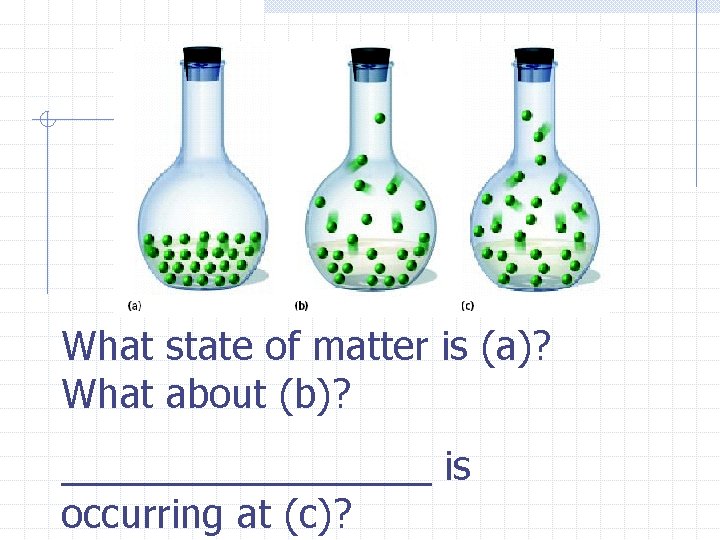
What state of matter is (a)? What about (b)? _________ is occurring at (c)?

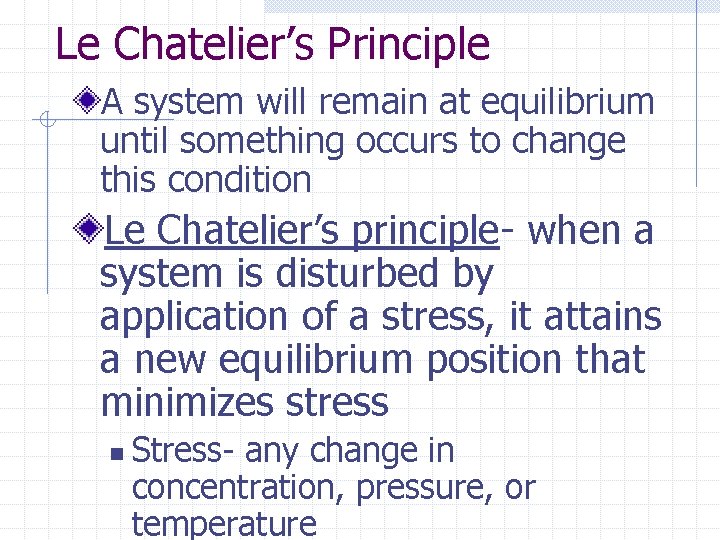
Le Chatelier’s Principle A system will remain at equilibrium until something occurs to change this condition Le Chatelier’s principle- when a system is disturbed by application of a stress, it attains a new equilibrium position that minimizes stress n Stress- any change in concentration, pressure, or temperature

Let’s look again at our equilibrium system… Liquid + heat energy vapor Which direction is the endothermic reaction? Which direction is the exothermic direction?

Equilibrium Shifts CHANGE Adding liquid Removing liquid Adding vapor Removing vapor Decrease volume Increase volume Decrease temperature Increase temperature SHIFT

Volatile and Nonvolatile Liquids Volatile liquids- liquids that evaporate readily Nonvolatile liquids- evaporate slowly, have strong attractive forces between particles

Boiling point-conversion of a liquid to a vapor within the liquid as well as at its surface.

Freezing and Melting Freezing point- the temperature at which the solid and liquid are in equilibrium at 1 atm pressure

Stop

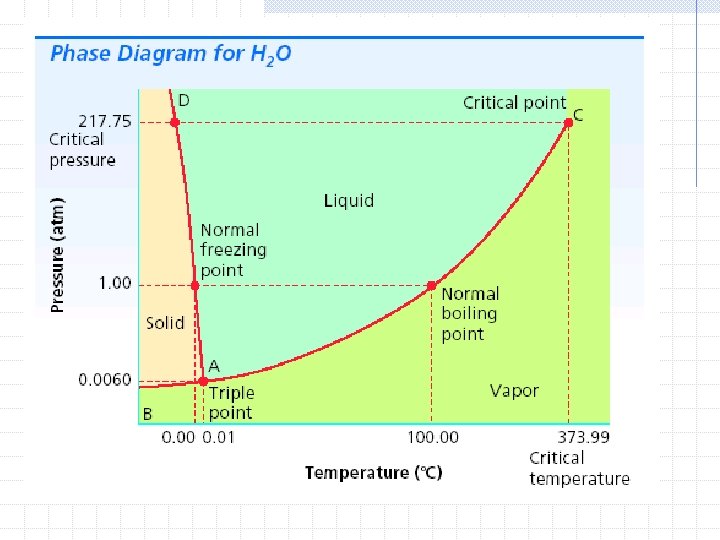
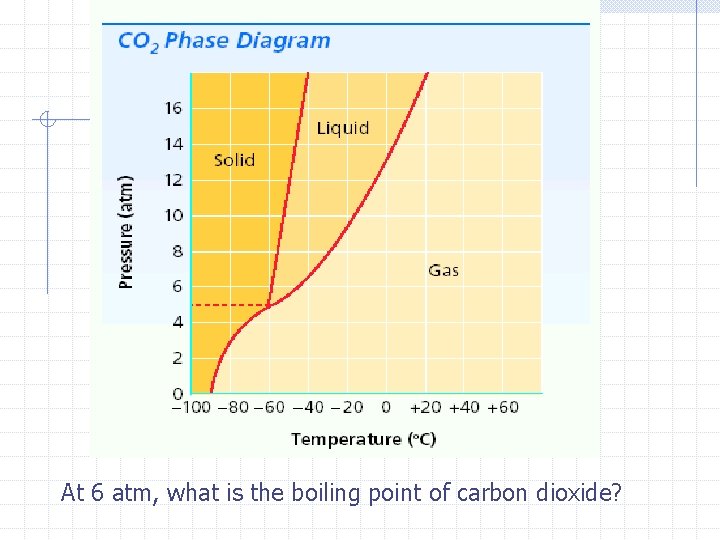
Phase Diagrams Phase diagrams- a graph of pressure versus temperature that shows the conditions under which the phases of a substance exist Triple point- indicates the temperature and pressure conditions at which the solid, liquid, and vapor of the substance can coexist at equilibrium

Critical temperature- the temperature above which the substance cannot exist in the liquid state n Water 373. 99°C Critical pressure- the lowest pressure at which the substance can exist as a liquid at the critical temperature n Water 217. 75 atm


At 6 atm, what is the boiling point of carbon dioxide?

Stop

Water is the Earth’s most abundant liquid n n Oceans, rivers, and lakes cover about 75% of the earth’s surface 70 -90% of the mass of living things is water

Structure of Water The molecules in solid or liquid water are linked by hydrogen bonding Ice consists of water molecules in a hexagonal arrangement. The empty spaces between molecules in this pattern accounts for the low density of ice Once the molecule is heated, water molecules can crowd closer together Thus, liquid water is denser than ice.

Physical Properties of Water Pure liquid water is transparent, odorless, tasteless, and almost colorless n Any observable impurities are caused by dissolved minerals, liquids, or gases It has a unique property of expanding in volume when it freezes n This explains why ice floats in water.


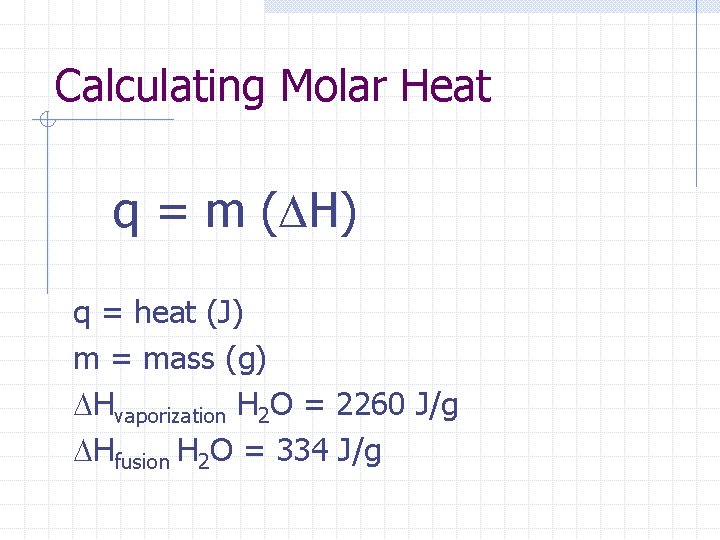
Molar heat of vaporization(ΔH) n It is a measure of the attraction between the particles of a liquid Molar heat of fusion ( H)Depends on the attraction between the solid particles

Calculating Molar Heat q = m ( H) q = heat (J) m = mass (g) Hvaporization H 2 O = 2260 J/g Hfusion H 2 O = 334 J/g

Practice Problems How much heat energy is absorbed when 47. 0 g of ice melts at STP? How much heat energy is absorbed when this same mass of liquid water boils? What quantity of heat energy is released when 506 g of liquid water freezes? What mass of steam is required to release 4. 97 x 105 k. J of heat energy on condensation?
 Solids liquids and gases section 2 properties of fluids
Solids liquids and gases section 2 properties of fluids Properties of solids and liquids
Properties of solids and liquids Properties of solids liquids and gases with examples
Properties of solids liquids and gases with examples Properties of solid liquid and gas
Properties of solid liquid and gas Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Chapter 14 solids liquids and gases
Chapter 14 solids liquids and gases Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids Expansion of solids liquids and gases examples
Expansion of solids liquids and gases examples States of matter venn diagram
States of matter venn diagram Process of liquid to gas
Process of liquid to gas Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Liquids and solids menu
Liquids and solids menu Lesson outline lesson 1 solids liquids and gases answer key
Lesson outline lesson 1 solids liquids and gases answer key Kesler science.com
Kesler science.com Particle movement in solids liquids and gases
Particle movement in solids liquids and gases How does sound travel through solids liquids and gases
How does sound travel through solids liquids and gases Motion of particles in solids, liquids and gases
Motion of particles in solids, liquids and gases Why are gases easy to compress?
Why are gases easy to compress? Theory of filtration
Theory of filtration Mechanical behavior of materials
Mechanical behavior of materials Properties of liquid in matter
Properties of liquid in matter Again
Again 12 properties of matter
12 properties of matter Properties of network covalent solids
Properties of network covalent solids Elastic properties of solids
Elastic properties of solids How to calculate volume
How to calculate volume Bulk properties of solids
Bulk properties of solids Extensive and intensive properties
Extensive and intensive properties Chemical and physical properties
Chemical and physical properties Pull examples
Pull examples Mother sauces names
Mother sauces names A yolk and cream mixture that is used to thicken liquids *
A yolk and cream mixture that is used to thicken liquids * Molecular theory of gases and liquids
Molecular theory of gases and liquids Example of pure liquid dielectric is
Example of pure liquid dielectric is Separating mixtures worksheet grade 5
Separating mixtures worksheet grade 5