GASES Chapter 13 The Nature of Gases Gases








































- Slides: 66

GASES Chapter 13

The Nature of Gases • Gases can be compressed by exerting pressure. • Gasses occupy the volume that they have available to them.

The Nature of Gases • Air was the first gas to be studied. • Composed of several different gases, but physical behavior is like that of a single gas. • Regardless of their chemical identity, all gases exhibit similar physical behaviors. • 1 mole of helium, oxygen, or methane, at STP occupy the exact same volume of 22. 4 L

The Nature of Gases • Most gases are made up of molecules – two or more atoms bonded together. • N 2, O 2, CO 2 , CH 4, Cl 2, H 2, Br 2, I 2, F 2 • Noble gases – He, Ne, Ar, Kr, Xe, Rn – do not bond with any other atoms under ordinary conditions. • Single atom - He, Ne • Diatomic molecules – N 2, O 2 • Polyatomic molecules – CO 2, CH 4

Physical Properties Common to all Gases: • 1. Gases have mass: • Density of a gas is much less than the density of a solid or liquid. • 2. It is easy to compress gases • 3. Gases fill their containers completely

Physical Properties Common to all Gases: • 4. Different gases can move through each other quite rapidly. • Diffusion – the movement of one substance through another. • 5. Gases exert pressure. • 6. The pressure of a gas depends on its temperature. • The higher the temperature of a gas, the higher the pressure.

Physical Properties Common to all Gases: • Gas properties are explained by a kinetic- molecular model that describes the behavior of submicroscopic particles that make up a gas.

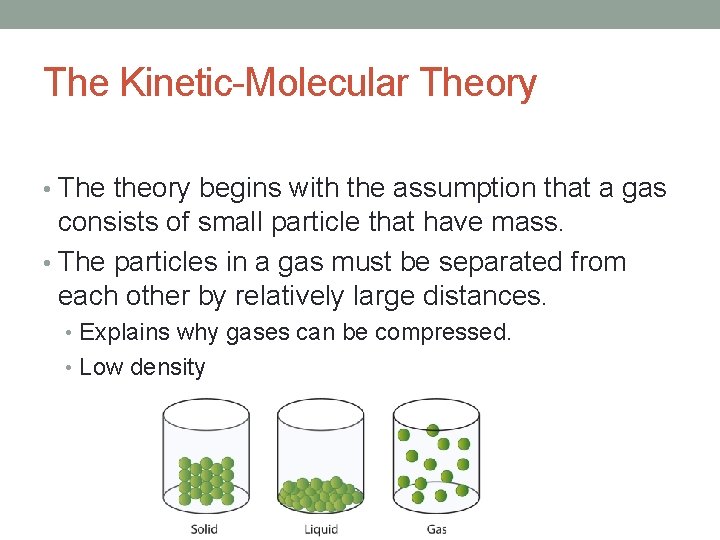
The Kinetic-Molecular Theory • The theory begins with the assumption that a gas consists of small particle that have mass. • The particles in a gas must be separated from each other by relatively large distances. • Explains why gases can be compressed. • Low density

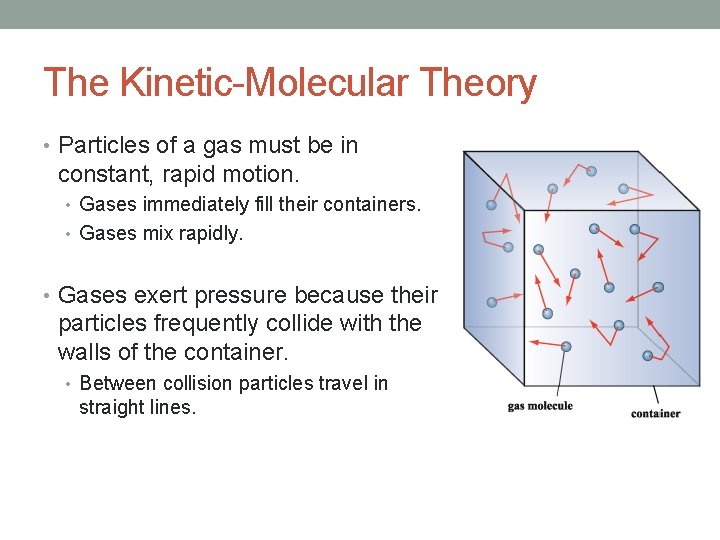
The Kinetic-Molecular Theory • Particles of a gas must be in constant, rapid motion. • Gases immediately fill their containers. • Gases mix rapidly. • Gases exert pressure because their particles frequently collide with the walls of the container. • Between collision particles travel in straight lines.

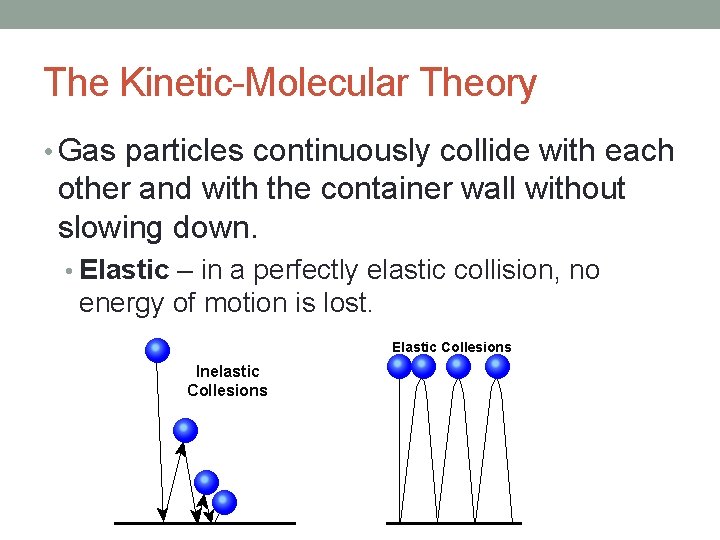
The Kinetic-Molecular Theory • Gas particles continuously collide with each other and with the container wall without slowing down. • Elastic – in a perfectly elastic collision, no energy of motion is lost.

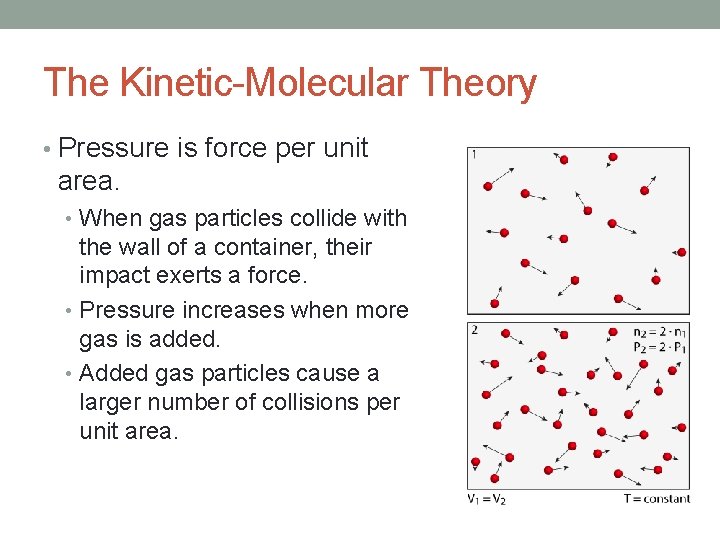
The Kinetic-Molecular Theory • Pressure is force per unit area. • When gas particles collide with the wall of a container, their impact exerts a force. • Pressure increases when more gas is added. • Added gas particles cause a larger number of collisions per unit area.

The Kinetic-Molecular Theory • Gas pressure increases when temperature is increased. • Temperature of a gas is a measure of the kinetic energy of the gas particle. • Kinetic energy – energy carried by objects in motion. • KE = mv 2/2 • m = mass of object • v = velocity • The higher the temperature of the gas, the higher the kinetic energy of its particles. • The gas particles speed up as the temperature changes.

The Kinetic-Molecular Theory • Gas exerts a greater pressure when its particles are moving faster. • The force with which the particles impact the walls of their container is proportional to their velocity. • At higher velocity the collisions are more forceful. • Gas particles also collide more frequently at higher velocities.

Kinetic-Molecular Theory • The kinetic-molecular theory was the work of three scientists: • Rudolf Clausius • James Clerk Maxwell • Ludwig Boltzmann

Kinetic-Molecular Theory • 1. A gas consists of very small particles, each of which has a mass. • 2. The distance separating gas particles are relatively large. • 3. Gas particles are in constant, rapid, random motion. • 4. Collisions of gas particles with each other or with the walls of the container are perfectly elastic. • 5. The average kinetic energy of gas particles depends only on the temperature of the gas. Higher energy at higher temperatures. • 6. Gas particles exert no attractive force on one another.

HOMEWORK Pg. 423 1 -6

MEASURING GASES 13 -2

Measuring Gases • Experimental work in chemistry requires the measurement of quantities such as volume, temperature, pressure, and amount of a sample. • Because gases are highly compressible, their volume depends on pressure, temperature and amount of the gas.

Measuring Gases • In order to describe a gas sample completely and then make predictions about its behavior under changed conditions, it is important to deal with the values of four variables – amount of gas, volume, temperature, and pressure.

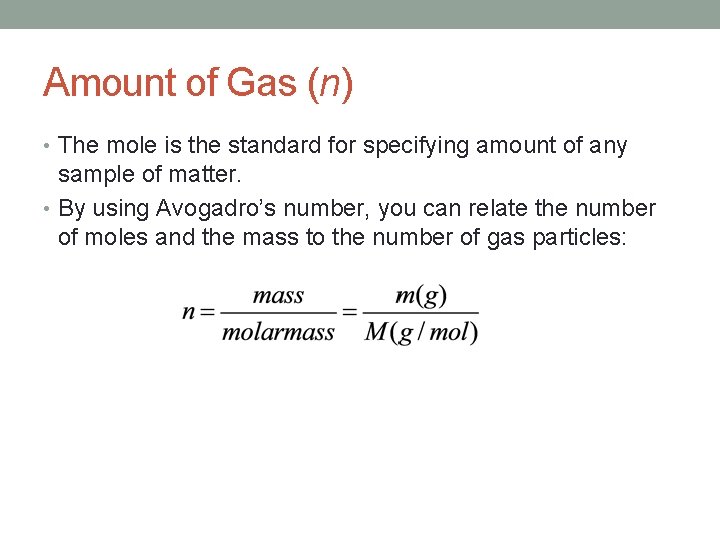
Amount of Gas (n) • The mole is the standard for specifying amount of any sample of matter. • By using Avogadro’s number, you can relate the number of moles and the mass to the number of gas particles:

Volume (V) • A gas uniformly fills any container in which it is placed. • Volume of the gas is the volume of its container. • Unit for volume = liter (L) • 1 ml = 10 dm 3 or 1000 dm 3 • 1 L = 10 cm 3 or 1000 cm 3

Temperature (T) • Temperature measured with a thermometer using degrees Celsius. • In calculation temperature needs to be converted to Kelvin. • SI unit • No negative temperatures. • Celsius to Kelvin conversion: T(K) = T(°C) + 273

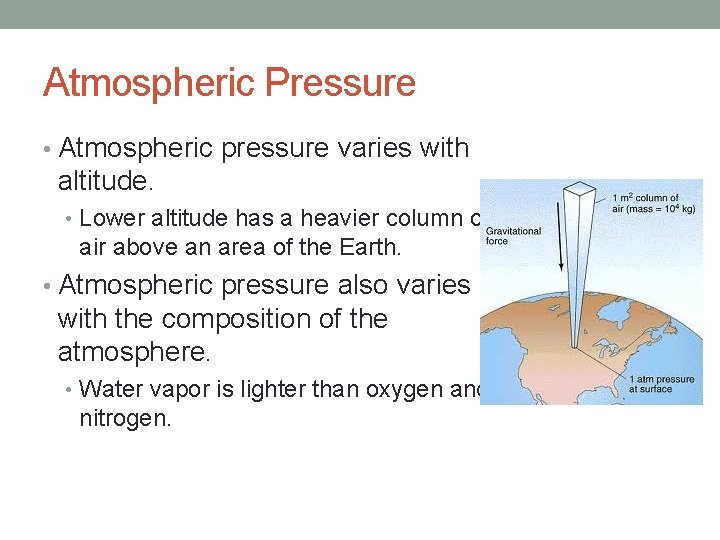
Atmospheric Pressure • Atmospheric pressure – pressure exerted by the air in the atmosphere. • Result of the fact that air has mass and is attracted by Earth’s gravity. • Pressure is calculated in units of force per unit area. • SI unit of force is the newton. • SI unit of pressure is the pascal. • atm – another unit of pressure. • Figure 13 -12

Atmospheric Pressure • Atmospheric pressure varies with altitude. • Lower altitude has a heavier column of air above an area of the Earth. • Atmospheric pressure also varies with the composition of the atmosphere. • Water vapor is lighter than oxygen and nitrogen.

Atmospheric Pressure Conversions • 1 atm = 760 mm Hg = 760 torr • 1 atm = 101. 3 k. Pa or 101325 Pa • 1 atm = 14. 70 lb/in² • 1 bar = 100000 Pa = 0. 9869 atm

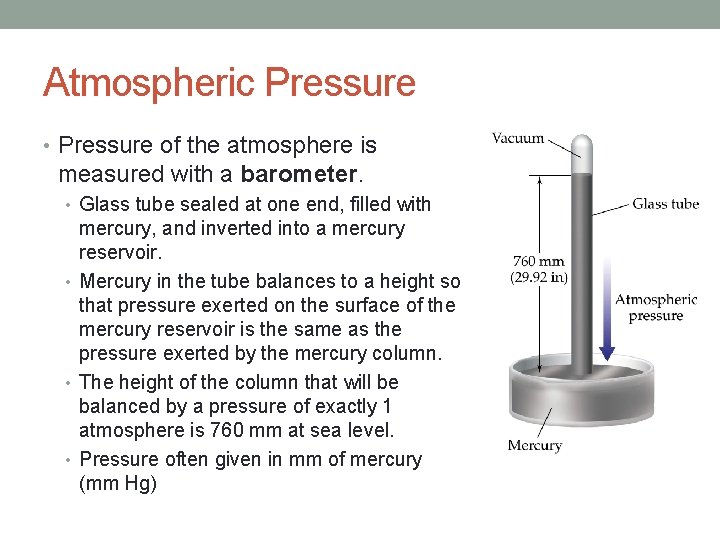
Atmospheric Pressure • Pressure of the atmosphere is measured with a barometer. • Glass tube sealed at one end, filled with mercury, and inverted into a mercury reservoir. • Mercury in the tube balances to a height so that pressure exerted on the surface of the mercury reservoir is the same as the pressure exerted by the mercury column. • The height of the column that will be balanced by a pressure of exactly 1 atmosphere is 760 mm at sea level. • Pressure often given in mm of mercury (mm Hg)

Practice Problems • The column of mercury in a barometer is 745 mm above the mercury reservoir at the bottom. What is the atmospheric pressure in pascals? • The air pressure inside the cabin of an airplane is 8. 3 lb/in 2 What is the pressure in atmospheric units?

Enclosed Gases • If a gas is in an open container, some of the gas will escape and pressure in the container will equal atmospheric pressure. • If the container is closed, pressure inside may differ from atmospheric pressure. • Measured with an instrument called a manometer • A U-shaped glass tube filled with mercury. • Determining the pressure in the container depends on whether the levels of mercury on the two sides are equal.

Enclosed Gases • If the levels of the mercury are the same on both sides, the pressure of the gas in the container is the same as atmospheric pressure. • If the level of mercury is lower on the container side, the pressure in the container is higher than atmospheric pressure. • The difference in pressure in units of mm. Hg is the difference between the heights of two mercury columns. • To find pressure in the container, add the difference to the atmospheric pressure.

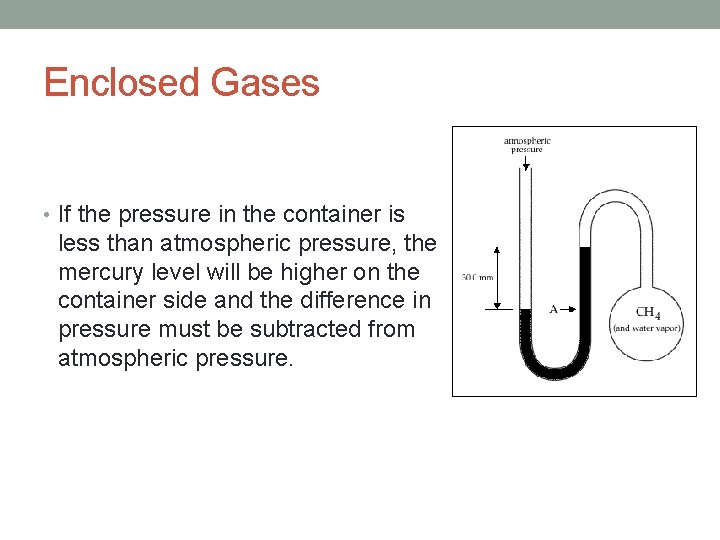
Enclosed Gases • If the pressure in the container is less than atmospheric pressure, the mercury level will be higher on the container side and the difference in pressure must be subtracted from atmospheric pressure.

Practice Problems • You have a closed container attached to a U-tube. The mercury in the open-ended side of the tube is higher, and the difference between the heights of the mercury column is 27 mm. You have measured the atmospheric pressure with a barometer to be 755 mm Hg. What is the pressure of the gas in the container in atmospheres?

STP • The behavior of a gas depends very strongly on the temperature and the pressure at which the gas is held. • STP – standard temperature and pressure. • The temperature that is designated as standard is 273 K • The pressure that is designated as standard is 1 atmosphere. • 1 atm = 760 mm Hg = 101, 325 Pa

HOMEWORK Pg. 4301 -4

THE GAS LAWS 13 -3

Boyle’s Law: The Pressure-Volume Relationship • Air is often used for its cushioning effect. • Gases can be used in this way because they can be compressed. • Robert Boyle - first one to note this property. • “Spring of air” • Boyle experimented with trapping a fixed amount of air, changing its pressure, and measuring its volume.

Boyle’s Law • Boyle altered the pressure and volume, but kept temperature and amount of gas constant. • Boyle wanted to answer the question: “Is there a relationship between the pressure and volume of a gas” • Figure 13 -16 • As pressure increases, volume decreases.

Boyle’s Law • Boyle’s Law – If the temperature of a given gas sample remains unchanged, the product of the pressure times the volume has a constant value. • Mathematical statement: PV = k 1 • P = pressure of the gas • V = volume of the gas • K 1 = the constant • Depends on units of P and V, the temperature and amount of gas. • The pressure and volume of a sample of gas at constant temperature are inversely proportional to each other.

Boyle’s Law • Boyle’s law allows us to calculate some quantities without having to measure them. • The law states that at the same temperature, the product of the pressure time the volume for a given sample of gas is always the same. • For two trials of different pressures: • P 1 V 1 = k 1 • P 2 V 2 = k 1 • Written as: P 1 V 1 = P 2 V 2

Practice Problems • A gas occupies a volume of 485 m. L at a pressure of 1. 01 k. Pa and temperature of 295 K. When the pressure is changed the volume becomes 477 m. L. Of there has been no change in temperature what is the new pressure? • A gas occupies a volume of 2. 45 L at a pressure of 1. 03 atm and temperature of 293 K What volume will the gas occupy if the pressure chages to 0. 980 atm and the temperature remains unchanged?

Charles’s Laws: The Temperature. Volume Relationship • Jacques Charles determined the relationship between the temperature and volume of a gas sample. • Experiment: Gas sample is trapped into a cylinder with a movable piston. • Temperature is altered by immersing the cylinder in water baths at different temperatures. • In a water bath with a different temperature the gas sample takes on a new volume. • Pressure and amount of gas were held constant.

Charles’s Law • Results: Figure 13 -19 • Volume increases as temperature increases. • The volume of a gas is directly proportional to its temperature. • Absolute temperature scale: • Zero at – 273. 15°C = absolute zero • Scale has only positive values: • Units = Kelvins (K) • Kelvins have the same magnitude as Celsius degrees: • K = °C + 273

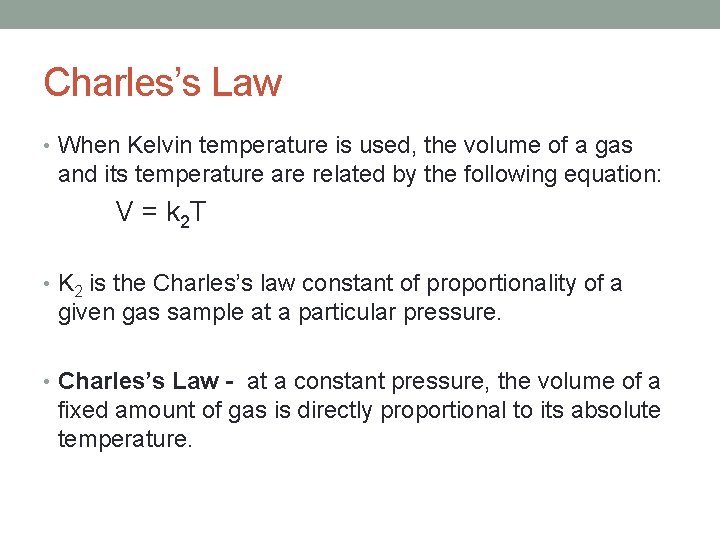
Charles’s Law • When Kelvin temperature is used, the volume of a gas and its temperature are related by the following equation: V = k 2 T • K 2 is the Charles’s law constant of proportionality of a given gas sample at a particular pressure. • Charles’s Law - at a constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature.

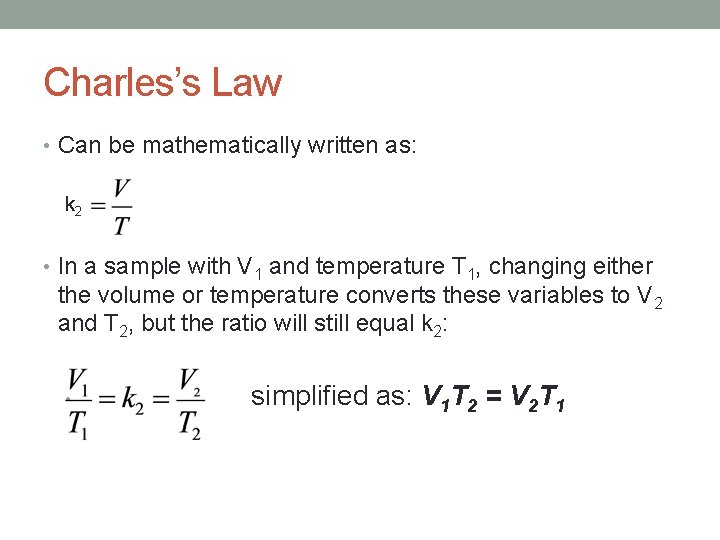
Charles’s Law • Can be mathematically written as: k 2 • In a sample with V 1 and temperature T 1, changing either the volume or temperature converts these variables to V 2 and T 2, but the ratio will still equal k 2: • simplified as: V 1 T 2 = V 2 T 1

Practice Problems • A hot-air balloon is filled with air at 5. 00°C until it is 80. 0 percent filled with air. When completely filled, the balloon has a volume of 1600. m 3. To what temperature would the air need to heated to fill the balloon completely? • What will be the volume of a gas sample at 309 K if its volume at 215 K is 3. 42 L? Assume that pressure is constant.

Avogadro’s Law: The Amount-Volume Relationship • Avogadro’s law states that equal volumes of gases at the same temperature and pressure contain an equal number of particles. • The law states that a gas with a larger volume must consists of a greater number of particles. • As long as pressure and temperature of a gas do not change, the only way to change the volume is by changing the number of gas particles: • V = k 3 n • Molar volume, volume of one mole, at STP = 22. 4 L

Dalton’s Law of Partial Pressure • Experimented with mixtures of gases. • He concluded that each gas in a mixture exerts the same pressure that is would if it were present alone at the same temperature. • The pressure exerted by each component of a mixture of gases is called the partial pressure of that component.

Dalton’s Law of Partial Pressures • Dalton’s law of partial pressure states that the sum of the partial pressure of all the components in a gas mixture is equal to the total pressure of the gas mixture. PT = pa + pb + pc + …. • PT = total pressure • pa, pb pc … = partial pressures of components

Practice Problems • What is the pressure of a mixtures of helium, nitrogen, and oxygen if their partial pressures are 600. mm Hg, 150. mm Hg, and 102 mm Hg. • A flask contains a mixture of hydrogen and oxygen. The pressure being exerted by these gases is 785 mm Hg. If the partial pressure of the hydrogen in the mixture is 395 mm Hg, what is the partial pressure of the oxygen.

HOMEWORK Pg. 440 1 -3

THE IDEAL GAS LAW 13 -4

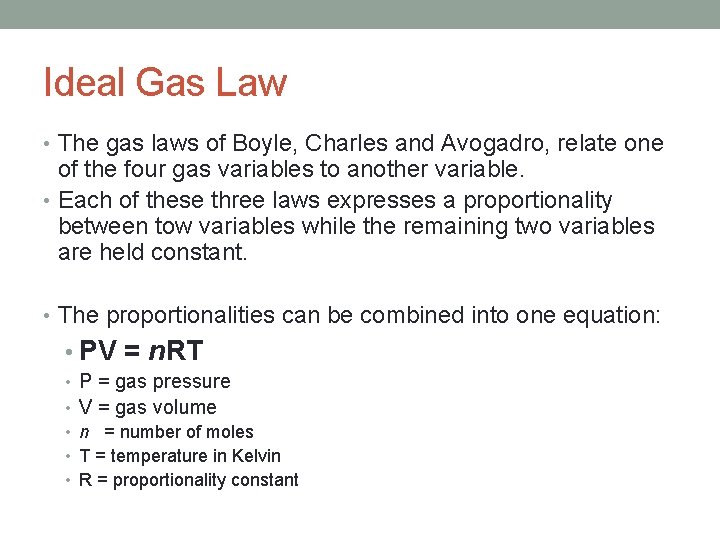
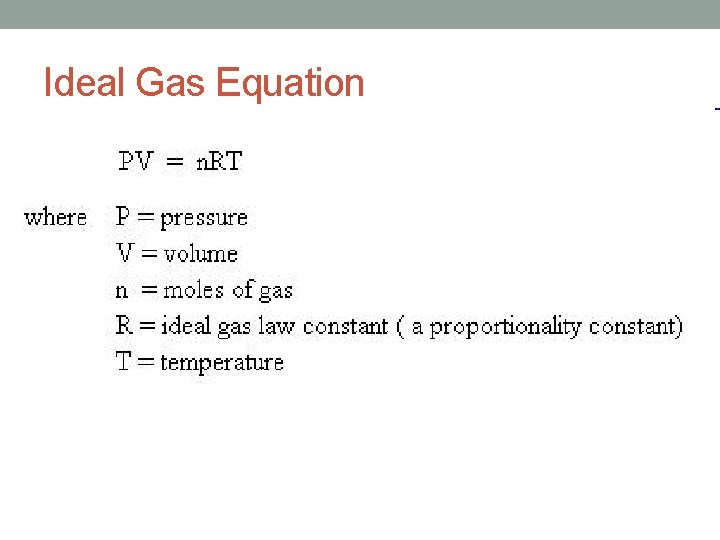
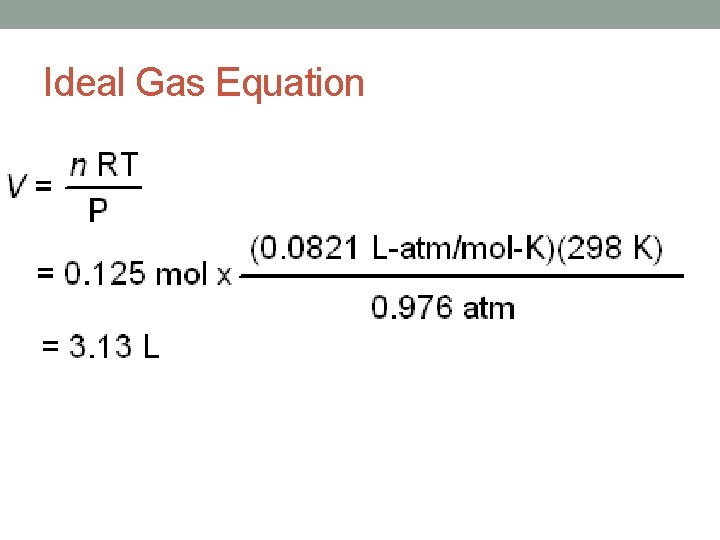
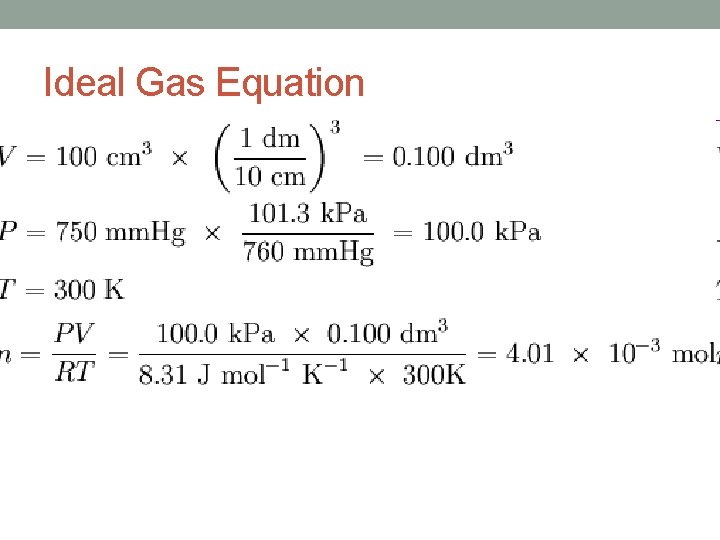
Ideal Gas Law • The gas laws of Boyle, Charles and Avogadro, relate one of the four gas variables to another variable. • Each of these three laws expresses a proportionality between tow variables while the remaining two variables are held constant. • The proportionalities can be combined into one equation: • PV = n. RT • P = gas pressure • V = gas volume • n = number of moles • T = temperature in Kelvin • R = proportionality constant

Ideal Gas Equation • The equation PV = n. Rt describes the physical behavior of an ideal gas in terms of the pressure, volume, temperature, and the number of moles of gas. • Ideal gas – a gas that is described by the kinetic- molecular theory postulates.

Ideal Gas Equation

Ideal Gas Equation

Ideal Gas Equation

Ideal Gas Law • R = gas constant • Numerical value depends its units. • 1. 00 mole of gas at 273 K and 1. 00 atm occupies a volume of 22. 4 L. • R = 0. 0821 atm-L/mol-K • R = 8. 31 dm 3 • k. Pa/mol • K

Practice Problems • If the pressure exerted by a gas at 0. 00°C in a volume of 0. 0010 L is 5. 00 atm, how many moles of gas are present? • What volume would be occupied by 100. g of oxygen gas at a pressure of 1. 50 atm a temperature of 25. 0°C?

Deviations from Ideal behavior • The ideal gas equation can be used to accurately predict gas behaviors in many situations. • It is an approximation and does not describe the behavior of real gases exactly. • Example: • Gases at very high pressure do not follow results given by the equation. • Gasses at very low temperature give values different from ones calculated by the equation.

Deviation from Ideal Behavior • An increase in pressure compresses the gas and particles move closer together. • As pressure increases more and more compression becomes difficult because gas particles have a volume of their own. • Kinetic-molecular theory assumes that gas particles have no volume so theory begins to fail.

Deviation from Ideal Behavior • As temperature decreases particles slow down. • Attractive forces between particles moving at high speed are negligibly small, but become significant as the particles slow down. • The kinetic-molecular model makes two false assumptions and so does not correspond exactly to all real situations. • The approximations are valid under most ordinary conditions and theory is still very useful.

HOMEWORK Pg. 445 1 -4

HOW GASES WORK 13 -5

Lifting Power of Gases • In order for a balloon to rise in air, it must weigh less than the air it is displacing. • Density of the gas needs to be much less than the density of air. • Gases that have potential as lifting gases include ammonia NH 3, methane CH 4, Hydrogen (H 2), and Helium (He). • Helium is the only safe option as it has no tendency to burn.

Lifting Power of Gases • The other method of lowering the density of a gas at constant pressure is to raise its temperature. • Hot-air balloons • The burner sends a stream heated air into the balloon. • Less expensive than helium and much safer than hydrogen. • Relatively weak lift.

Gas Effusion • Effusion – the movement of atoms or molecules through tiny spaces where only one particle can pass at a time. • Some gases effuse faster than others. • Hydrogen effuses faster than helium. • Helium effuses faster than oxygen. • Lighter gases effuse and diffuse faster than heavier gases because, at a given temperature, the particles of the lighter gas have greater speeds than the particles of the heavier gas.

HOMEWORK Pg. 449 1 -3
 State boyle’s law.
State boyle’s law. Nature and nature's law lay hid in night
Nature and nature's law lay hid in night Determinace lidské psychiky
Determinace lidské psychiky Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worm breton
Tư thế worm breton Chúa yêu trần thế
Chúa yêu trần thế Các môn thể thao bắt đầu bằng tiếng nhảy
Các môn thể thao bắt đầu bằng tiếng nhảy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Cong thức tính động năng
Cong thức tính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Ví dụ về giọng cùng tên
Ví dụ về giọng cùng tên Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dot
Dot Bảng số nguyên tố
Bảng số nguyên tố Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Avogadro's law relationship
Avogadro's law relationship Chapter 11 review gases section 1
Chapter 11 review gases section 1 Charles' law worksheet answers
Charles' law worksheet answers Chapter 14 behavior of gases
Chapter 14 behavior of gases Section 13.2 the combined gas law and avogadro's principle
Section 13.2 the combined gas law and avogadro's principle Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Chapter 14 solids liquids and gases
Chapter 14 solids liquids and gases Nature of nurture chapter 3
Nature of nurture chapter 3 Nature of nurture chapter 3
Nature of nurture chapter 3 For or against
For or against Strategic management chapter 1
Strategic management chapter 1 Chapter 10 lipids nature's flavor enhancers
Chapter 10 lipids nature's flavor enhancers Section 1 the nature of energy
Section 1 the nature of energy Nature of nurture chapter 3
Nature of nurture chapter 3 Nature of science chapter 1
Nature of science chapter 1 Chapter 2 lesson 1 the nature of matter
Chapter 2 lesson 1 the nature of matter Non examples of homogeneous mixture
Non examples of homogeneous mixture Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Basic food chemistry the nature of matter
Basic food chemistry the nature of matter The nature of matter chapter 2
The nature of matter chapter 2 Nature of nurture chapter 2
Nature of nurture chapter 2 Section 3 communicating with graphs answer key
Section 3 communicating with graphs answer key