Chapter 12 Chemical Kinetics Chemical Kinetics Studies the



![a. A + b. B products Trial [A] M/S [B] M/S Rate M/S 1 a. A + b. B products Trial [A] M/S [B] M/S Rate M/S 1](https://slidetodoc.com/presentation_image/0652e814b0a6e43b42da8440b38281ab/image-17.jpg)
![Concentration and Rate Homework Compare Experiments 1 and 2: when [NH 4+] doubles, the Concentration and Rate Homework Compare Experiments 1 and 2: when [NH 4+] doubles, the](https://slidetodoc.com/presentation_image/0652e814b0a6e43b42da8440b38281ab/image-18.jpg)
![Concentration and Rate Homework Likewise, compare Experiments 5 and 6: when [NO 2 -] Concentration and Rate Homework Likewise, compare Experiments 5 and 6: when [NO 2 -]](https://slidetodoc.com/presentation_image/0652e814b0a6e43b42da8440b38281ab/image-20.jpg)



![Zero Order Integration 2 NH 3(g) N 2 + 3 H 2 (g) [A]t Zero Order Integration 2 NH 3(g) N 2 + 3 H 2 (g) [A]t](https://slidetodoc.com/presentation_image/0652e814b0a6e43b42da8440b38281ab/image-28.jpg)
![[NO 2] vs Time • The plot is not a straight line, so the [NO 2] vs Time • The plot is not a straight line, so the](https://slidetodoc.com/presentation_image/0652e814b0a6e43b42da8440b38281ab/image-32.jpg)
![Determining rxn order Graphing ln [NO 2] vs. t yields: • The plot is Determining rxn order Graphing ln [NO 2] vs. t yields: • The plot is](https://slidetodoc.com/presentation_image/0652e814b0a6e43b42da8440b38281ab/image-33.jpg)
![Second-Order Processes A graph of 1/[NO 2] vs. t gives this plot. Time (s) Second-Order Processes A graph of 1/[NO 2] vs. t gives this plot. Time (s)](https://slidetodoc.com/presentation_image/0652e814b0a6e43b42da8440b38281ab/image-34.jpg)





- Slides: 51

Chapter 12 Chemical Kinetics

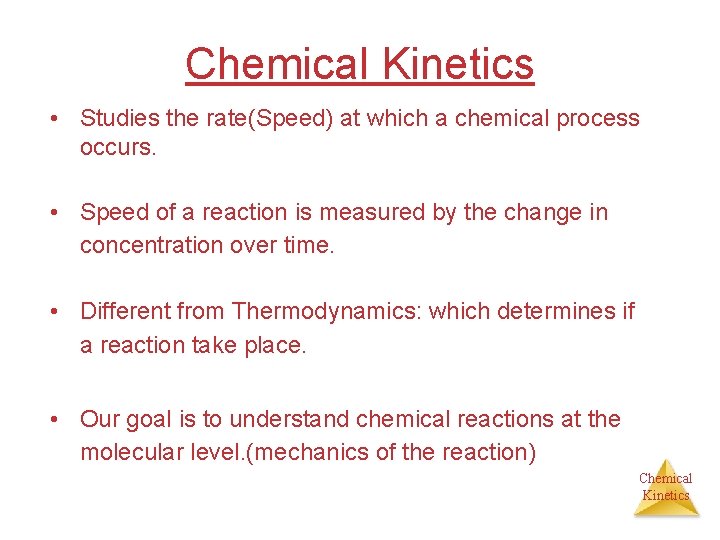
Chemical Kinetics • Studies the rate(Speed) at which a chemical process occurs. • Speed of a reaction is measured by the change in concentration over time. • Different from Thermodynamics: which determines if a reaction take place. • Our goal is to understand chemical reactions at the molecular level. (mechanics of the reaction) Chemical Kinetics

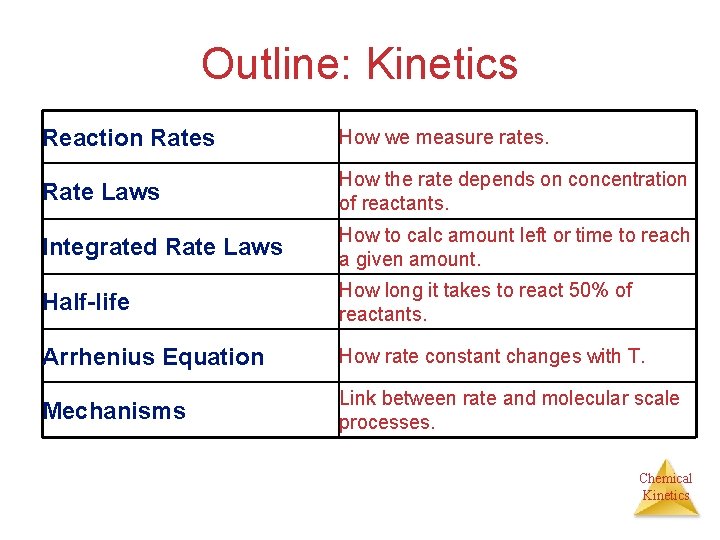
Outline: Kinetics Reaction Rates How we measure rates. Rate Laws How the rate depends on concentration of reactants. Integrated Rate Laws How to calc amount left or time to reach a given amount. Half-life How long it takes to react 50% of reactants. Arrhenius Equation How rate constant changes with T. Mechanisms Link between rate and molecular scale processes. Chemical Kinetics

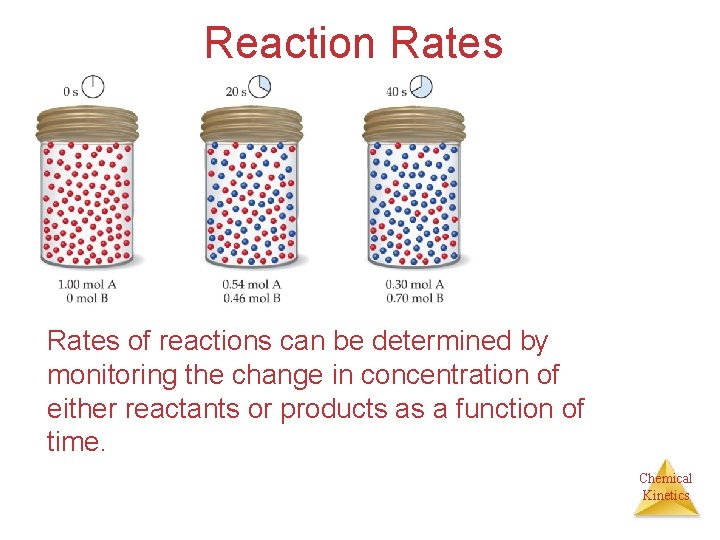
Reaction Rates • We define the reaction rate as the change in concentration of a reactant or product per unit time. • For the reaction “A measuring rate: B” there are two ways of (1) the speed at which the reactants disappear (2) the speed at which the products appear Chemical Kinetics

Reaction Rates of reactions can be determined by monitoring the change in concentration of either reactants or products as a function of time. Chemical Kinetics

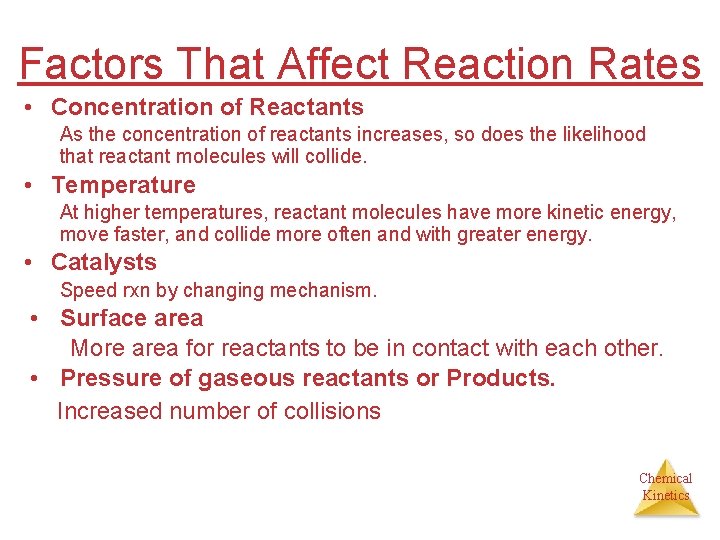
Factors That Affect Reaction Rates • Concentration of Reactants As the concentration of reactants increases, so does the likelihood that reactant molecules will collide. • Temperature At higher temperatures, reactant molecules have more kinetic energy, move faster, and collide more often and with greater energy. • Catalysts Speed rxn by changing mechanism. • Surface area More area for reactants to be in contact with each other. • Pressure of gaseous reactants or Products. Increased number of collisions Chemical Kinetics

Chemical Kinetics

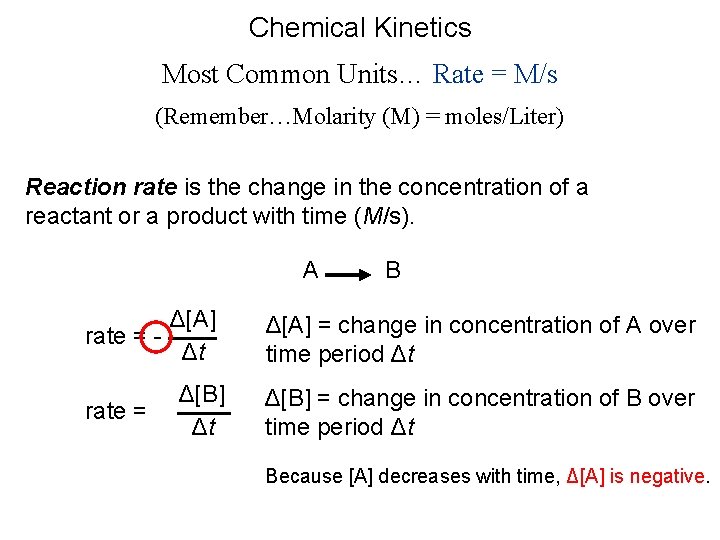
Chemical Kinetics Most Common Units… Rate = M/s (Remember…Molarity (M) = moles/Liter) Reaction rate is the change in the concentration of a reactant or a product with time (M/s). A Δ[A] rate = Δt rate = Δ[B] Δt B Δ[A] = change in concentration of A over time period Δt Δ[B] = change in concentration of B over time period Δt Because [A] decreases with time, Δ[A] is negative.

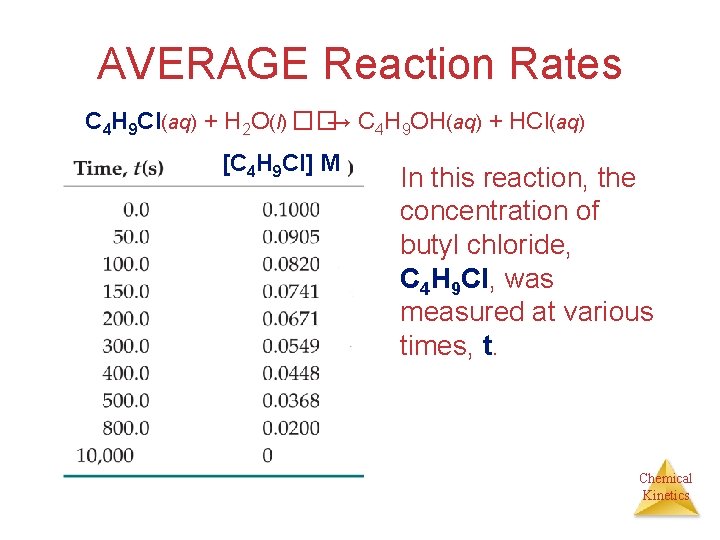
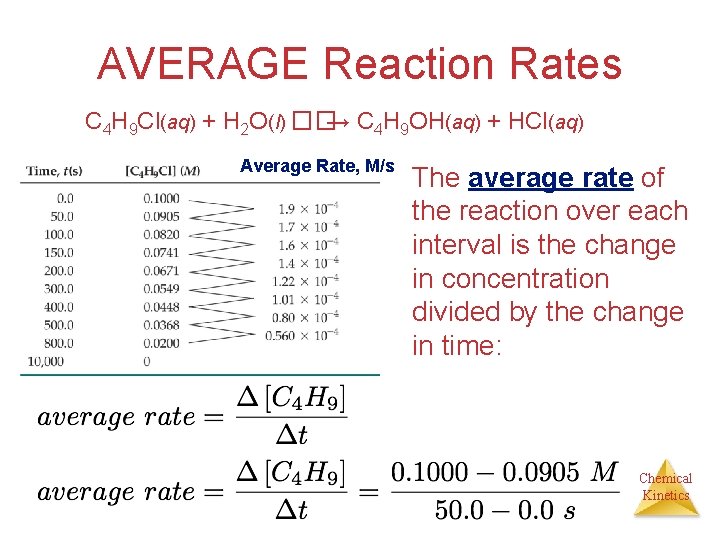
AVERAGE Reaction Rates C 4 H 9 Cl(aq) + H 2 O(l) ��→ C 4 H 9 OH(aq) + HCl(aq) [C 4 H 9 Cl] M In this reaction, the concentration of butyl chloride, C 4 H 9 Cl, was measured at various times, t. Chemical Kinetics

AVERAGE Reaction Rates C 4 H 9 Cl(aq) + H 2 O(l) ��→ C 4 H 9 OH(aq) + HCl(aq) Average Rate, M/s The average rate of the reaction over each interval is the change in concentration divided by the change in time: Chemical Kinetics

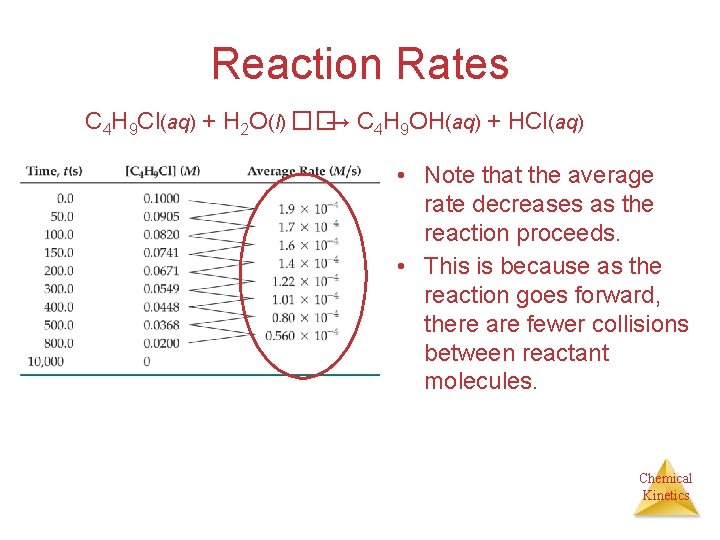
Reaction Rates C 4 H 9 Cl(aq) + H 2 O(l) ��→ C 4 H 9 OH(aq) + HCl(aq) • Note that the average rate decreases as the reaction proceeds. • This is because as the reaction goes forward, there are fewer collisions between reactant molecules. Chemical Kinetics

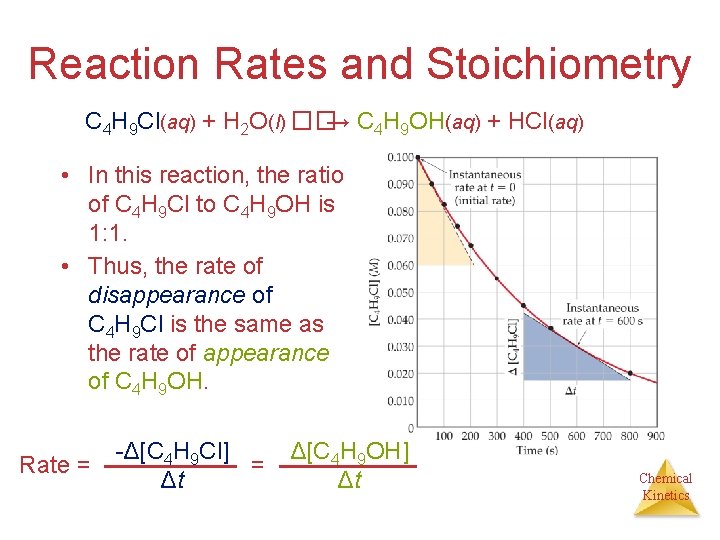
Reaction Rates and Stoichiometry C 4 H 9 Cl(aq) + H 2 O(l) ��→ C 4 H 9 OH(aq) + HCl(aq) • In this reaction, the ratio of C 4 H 9 Cl to C 4 H 9 OH is 1: 1. • Thus, the rate of disappearance of C 4 H 9 Cl is the same as the rate of appearance of C 4 H 9 OH. Rate = -Δ[C 4 H 9 Cl] = Δt Δ[C 4 H 9 OH] Δt Chemical Kinetics

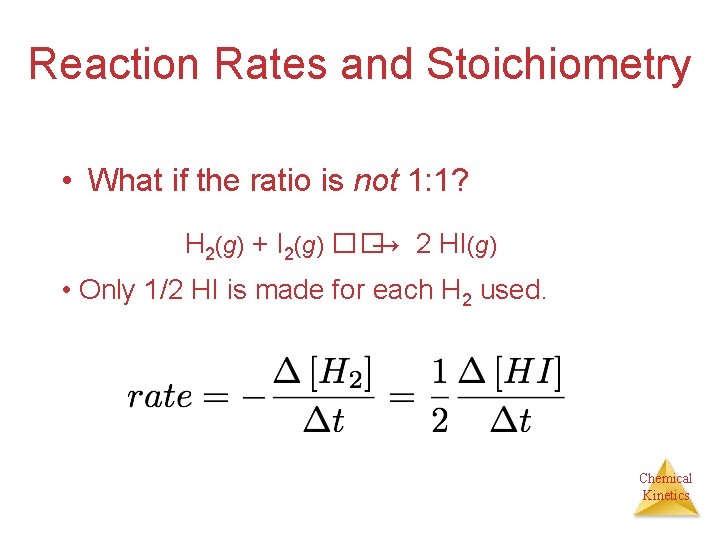
Reaction Rates and Stoichiometry • What if the ratio is not 1: 1? H 2(g) + I 2(g) ��→ 2 HI(g) • Only 1/2 HI is made for each H 2 used. Chemical Kinetics

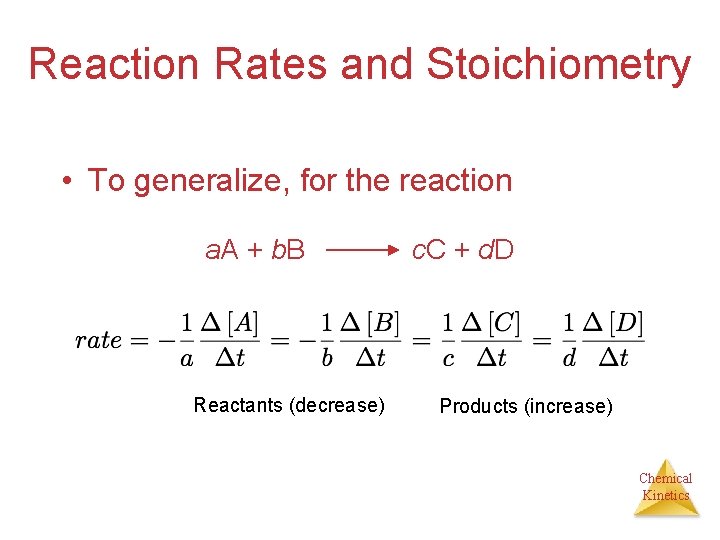
Reaction Rates and Stoichiometry • To generalize, for the reaction a. A + b. B Reactants (decrease) c. C + d. D Products (increase) Chemical Kinetics

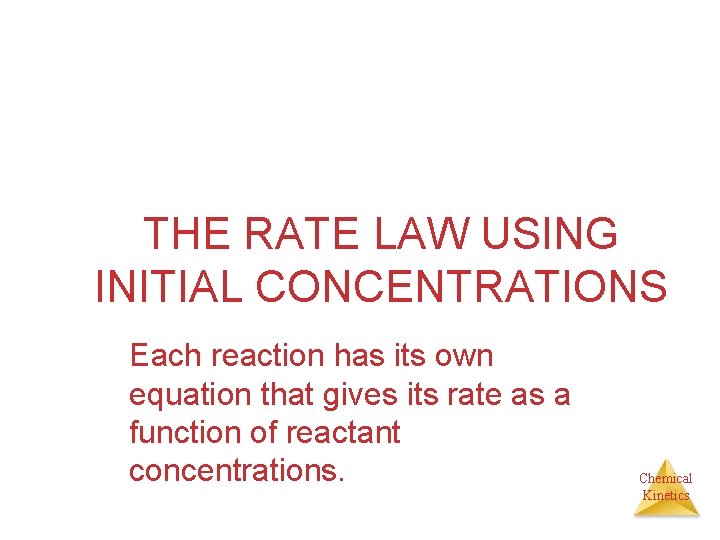
THE RATE LAW USING INITIAL CONCENTRATIONS Each reaction has its own equation that gives its rate as a function of reactant concentrations. Chemical Kinetics

Calculating The Initial Rate Law The rate law expresses the mathematical relationship of the rate of a reaction to the concentrations of the reactants. The products play no role in the calculations. a. A + b. B products Rate = k [A] x [B] y Rate = Molarity /sec k= rate constant and it varies with each reaction, temperature dependent. [A] [B] = Concentrations of reactants in M (moles/liter) x and y = Values for the reaction order. They tell us how important each of the reactants is in regard to speed. The higher the number the more value it has to overall speed. x does not equal a and y does not equal b
![a A b B products Trial A MS B MS Rate MS 1 a. A + b. B products Trial [A] M/S [B] M/S Rate M/S 1](https://slidetodoc.com/presentation_image/0652e814b0a6e43b42da8440b38281ab/image-17.jpg)
a. A + b. B products Trial [A] M/S [B] M/S Rate M/S 1 0. 100 2. 00 x 10 -3 2 0. 200 . 100 4. 00 x 10 -3 3 0. 200 16. 00 x 10 -3
![Concentration and Rate Homework Compare Experiments 1 and 2 when NH 4 doubles the Concentration and Rate Homework Compare Experiments 1 and 2: when [NH 4+] doubles, the](https://slidetodoc.com/presentation_image/0652e814b0a6e43b42da8440b38281ab/image-18.jpg)
Concentration and Rate Homework Compare Experiments 1 and 2: when [NH 4+] doubles, the initial rate doubles. Chemical Kinetics

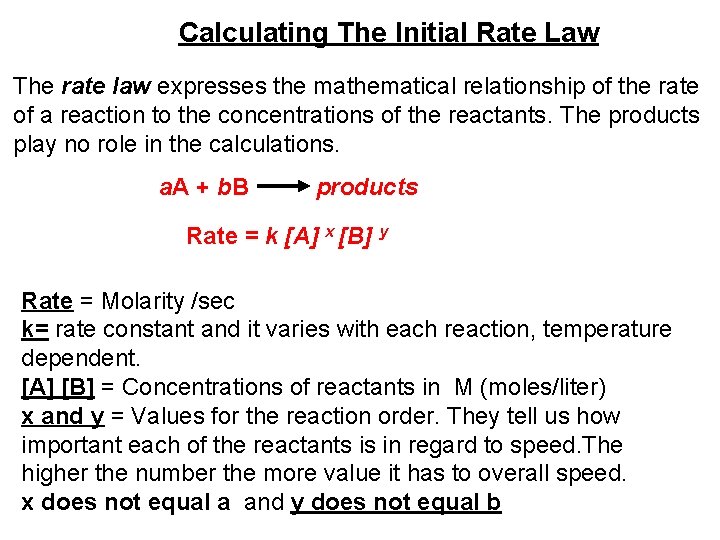
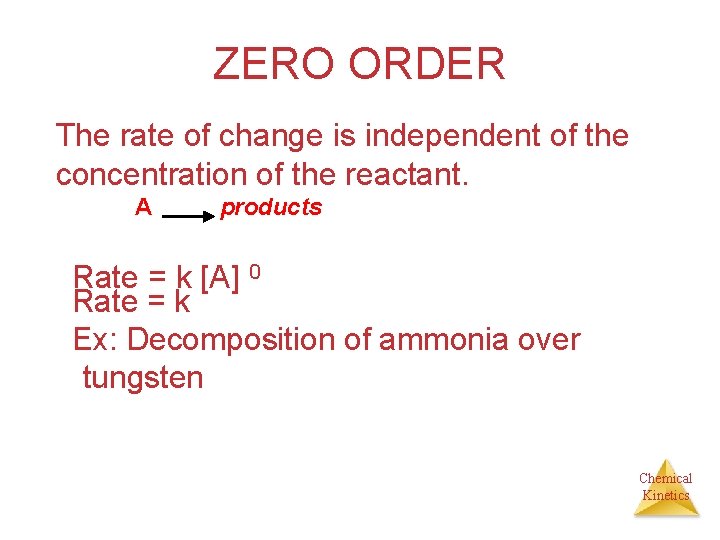
ZERO ORDER The rate of change is independent of the concentration of the reactant. A products Rate = k [A] 0 Rate = k Ex: Decomposition of ammonia over tungsten Chemical Kinetics
![Concentration and Rate Homework Likewise compare Experiments 5 and 6 when NO 2 Concentration and Rate Homework Likewise, compare Experiments 5 and 6: when [NO 2 -]](https://slidetodoc.com/presentation_image/0652e814b0a6e43b42da8440b38281ab/image-20.jpg)
Concentration and Rate Homework Likewise, compare Experiments 5 and 6: when [NO 2 -] doubles, the initial rate doubles. Chemical Kinetics

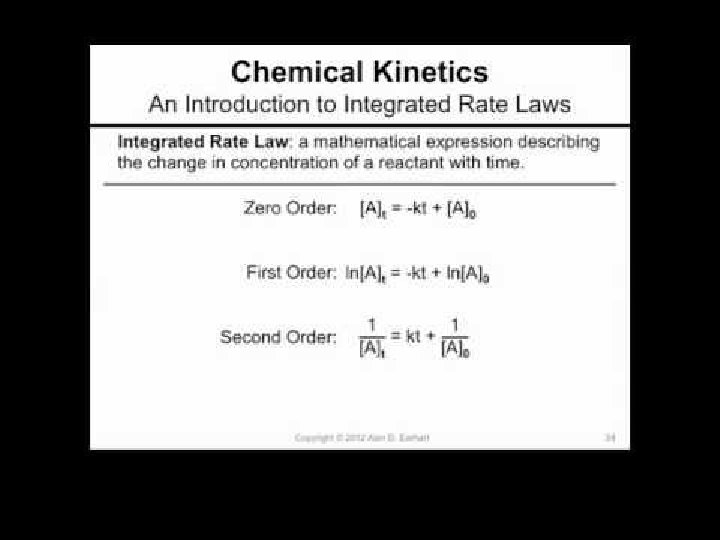
INTEGRATED RATE LAWS Chemical Kinetics

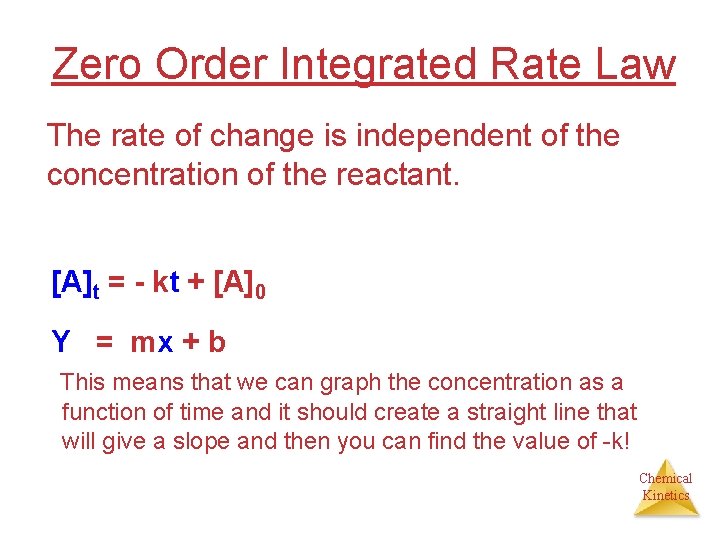
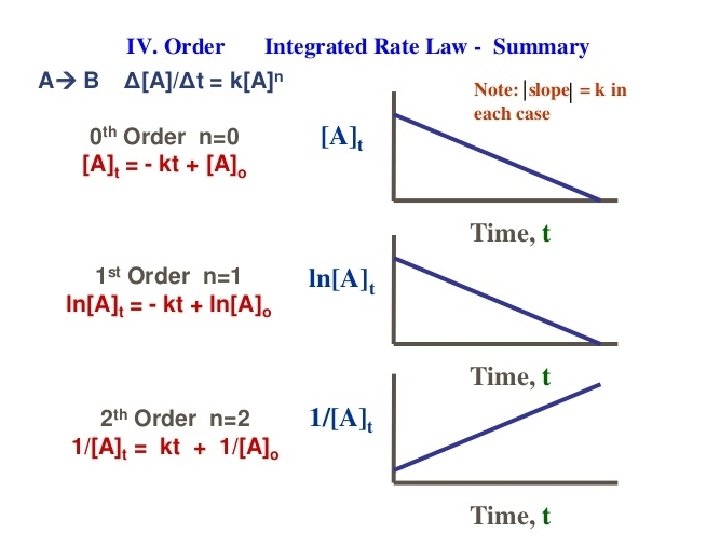
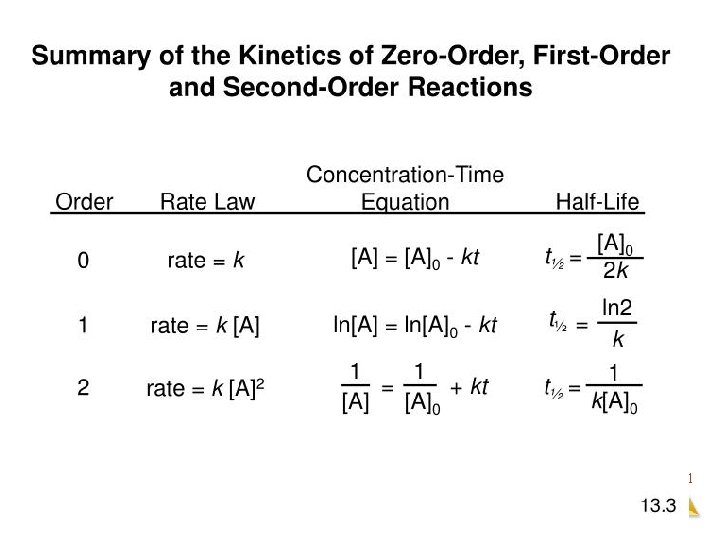
Zero Order Integrated Rate Law The rate of change is independent of the concentration of the reactant. [A]t = - kt + [A]0 Y = mx + b This means that we can graph the concentration as a function of time and it should create a straight line that will give a slope and then you can find the value of -k! Chemical Kinetics

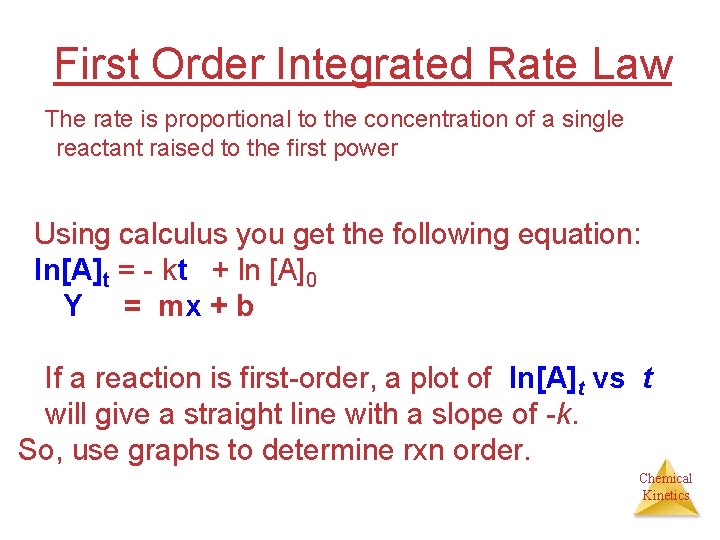
First Order Integrated Rate Law The rate is proportional to the concentration of a single reactant raised to the first power Using calculus you get the following equation: ln[A]t = - kt + ln [A]0 Y = mx + b If a reaction is first-order, a plot of ln[A]t vs t will give a straight line with a slope of -k. So, use graphs to determine rxn order. Chemical Kinetics

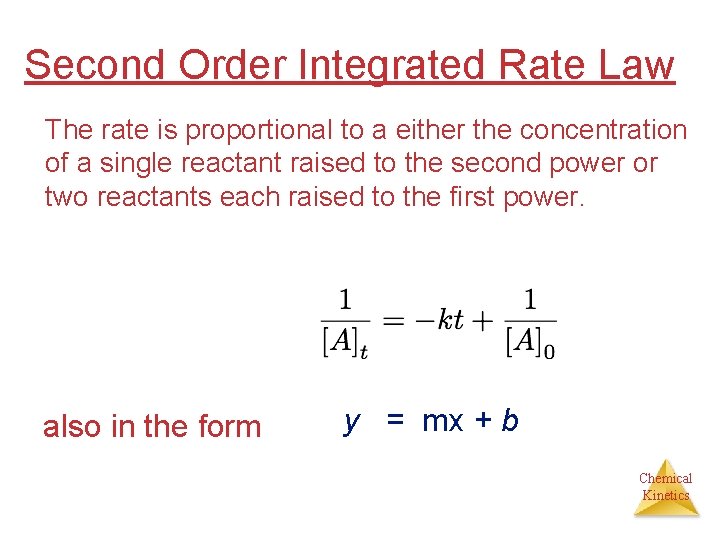
Second Order Integrated Rate Law The rate is proportional to a either the concentration of a single reactant raised to the second power or two reactants each raised to the first power. also in the form y = mx + b Chemical Kinetics

How are these equations helpful? • You now have a technique for determining the order of a reaction by observing graphs created from experimental data of concentration vs time. • If a simple graph of concentration vs time is a straight line then you know that it is a zero order reaction. • If the natural log of concentration vs time is a straight line then you know that it is a first order reaction. • If the reciprocal concentration vs time is a straight line then you know that it is a second order. Chemical Kinetics

The conclusion: In all cases, once you get a straight line fit , you can then get the rate constant from calculating the slope of the line. Slope (m) = ΔY ΔX Chemical Kinetics

Chemical Kinetics
![Zero Order Integration 2 NH 3g N 2 3 H 2 g At Zero Order Integration 2 NH 3(g) N 2 + 3 H 2 (g) [A]t](https://slidetodoc.com/presentation_image/0652e814b0a6e43b42da8440b38281ab/image-28.jpg)
Zero Order Integration 2 NH 3(g) N 2 + 3 H 2 (g) [A]t = - kt + [A]0 Chemical Kinetics

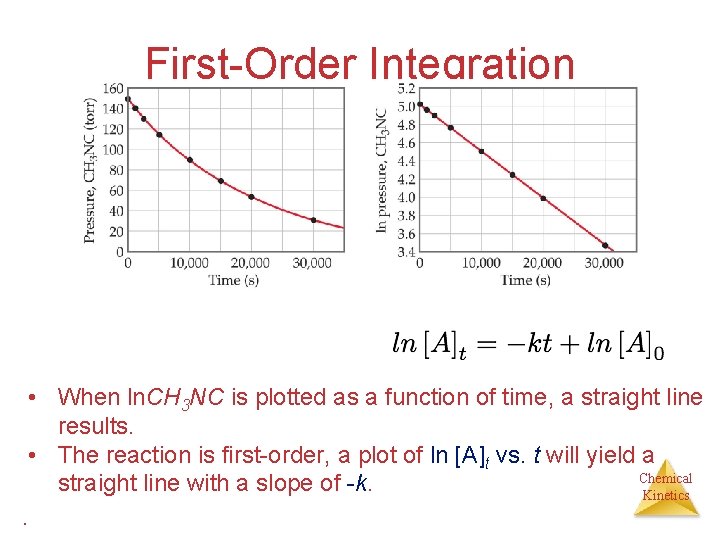
First-Order Integration • When ln. CH 3 NC is plotted as a function of time, a straight line results. • The reaction is first-order, a plot of ln [A]t vs. t will yield a Chemical straight line with a slope of -k. Kinetics.

Second-Order Integration So if a reaction is second-order in A, a plot of vs. t will yield a straight line with a slope of k. Chemical Kinetics

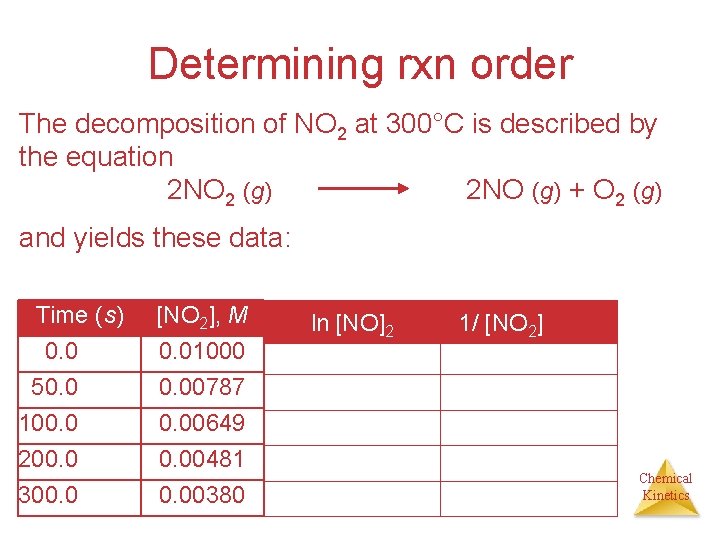
Determining rxn order The decomposition of NO 2 at 300°C is described by the equation 2 NO 2 (g) 2 NO (g) + O 2 (g) and yields these data: Time (s) 0. 0 50. 0 100. 0 200. 0 300. 0 [NO 2], M 0. 01000 0. 00787 0. 00649 0. 00481 0. 00380 ln [NO]2 1/ [NO 2] Chemical Kinetics
![NO 2 vs Time The plot is not a straight line so the [NO 2] vs Time • The plot is not a straight line, so the](https://slidetodoc.com/presentation_image/0652e814b0a6e43b42da8440b38281ab/image-32.jpg)
[NO 2] vs Time • The plot is not a straight line, so the process is not zero-order. Chemical Kinetics
![Determining rxn order Graphing ln NO 2 vs t yields The plot is Determining rxn order Graphing ln [NO 2] vs. t yields: • The plot is](https://slidetodoc.com/presentation_image/0652e814b0a6e43b42da8440b38281ab/image-33.jpg)
Determining rxn order Graphing ln [NO 2] vs. t yields: • The plot is not a straight line, so the process is not first-order in [A]. Time (s) 0. 0 50. 0 100. 0 200. 0 300. 0 [NO 2], M 0. 01000 0. 00787 0. 00649 0. 00481 0. 00380 ln [NO 2] -4. 610 -4. 845 -5. 038 -5. 337 -5. 573 Does not fit: Chemical Kinetics
![SecondOrder Processes A graph of 1NO 2 vs t gives this plot Time s Second-Order Processes A graph of 1/[NO 2] vs. t gives this plot. Time (s)](https://slidetodoc.com/presentation_image/0652e814b0a6e43b42da8440b38281ab/image-34.jpg)
Second-Order Processes A graph of 1/[NO 2] vs. t gives this plot. Time (s) 0. 0 50. 0 100. 0 200. 0 300. 0 [NO 2], M 0. 01000 0. 00787 0. 00649 0. 00481 0. 00380 1/[NO 2] 100 127 154 208 263 • This is a straight line. Therefore, the process is secondorder in [NO 2]. k= 0. 543 Chemical Kinetics

Chemical Kinetics

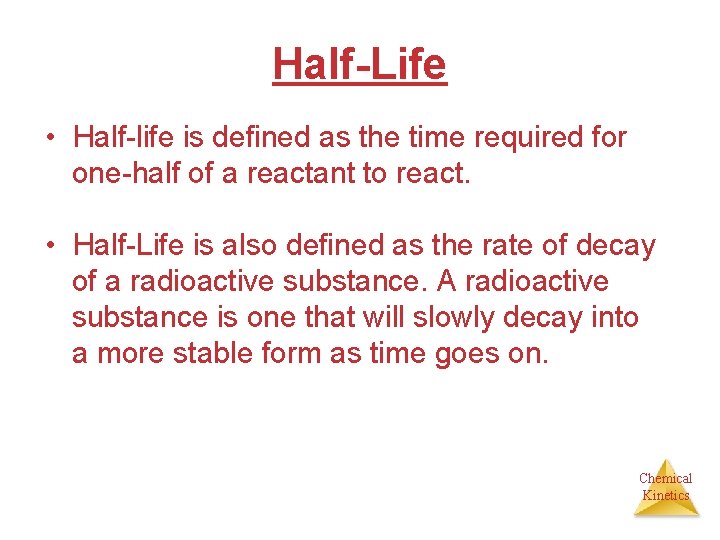
Half-Life • Half-life is defined as the time required for one-half of a reactant to react. • Half-Life is also defined as the rate of decay of a radioactive substance. A radioactive substance is one that will slowly decay into a more stable form as time goes on. Chemical Kinetics

Determining a Half Life To determine a half life, t½, the time required for the initial concentration of a reactant to be reduced to one-half its initial value, we need to know: ● The order of the reaction or enough information to determine it. ● The rate constant, k, for the reaction or enough information to determine it. ● In some cases, we need to know the initial concentration, [Ao] ● Substitute this information into the equation for the half life of a reaction with this order and solve for t½. Chemical Kinetics

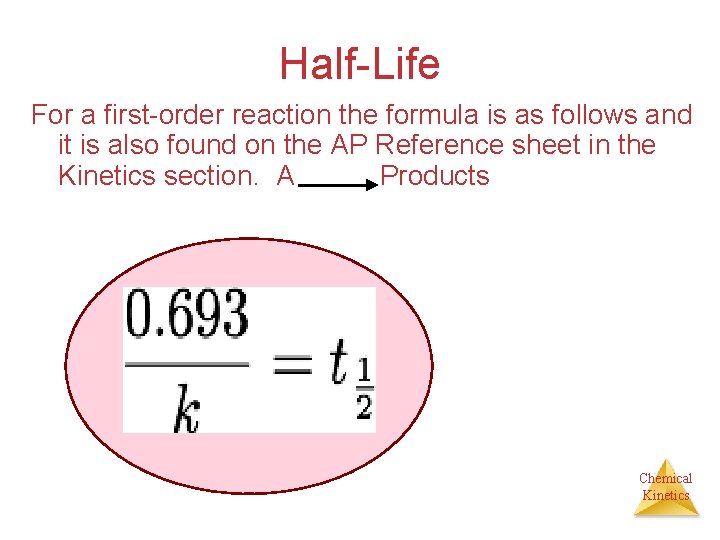
Half-Life For a first-order reaction the formula is as follows and it is also found on the AP Reference sheet in the Kinetics section. A Products Chemical Kinetics

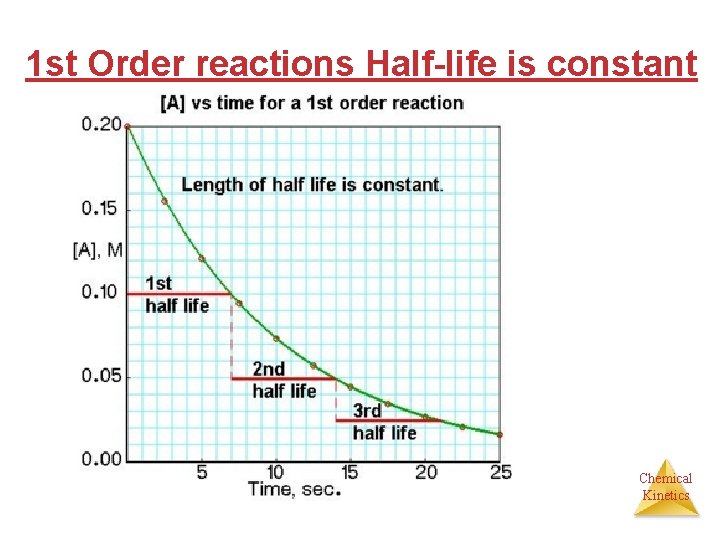
1 st Order reactions Half-life is constant Chemical Kinetics

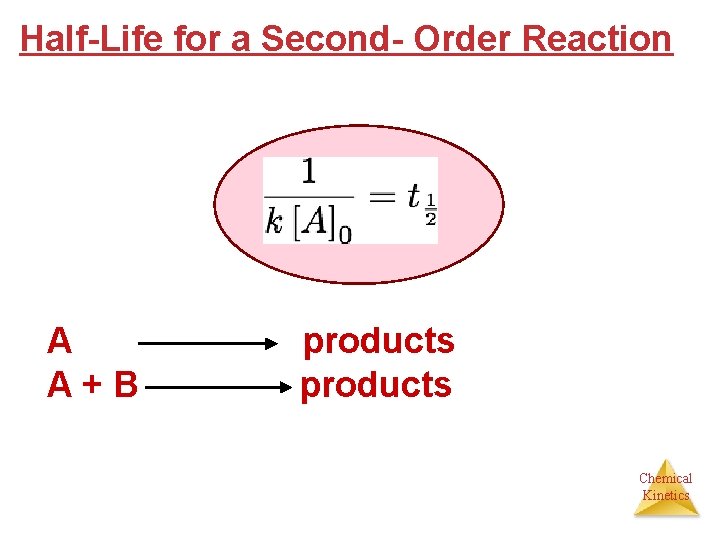
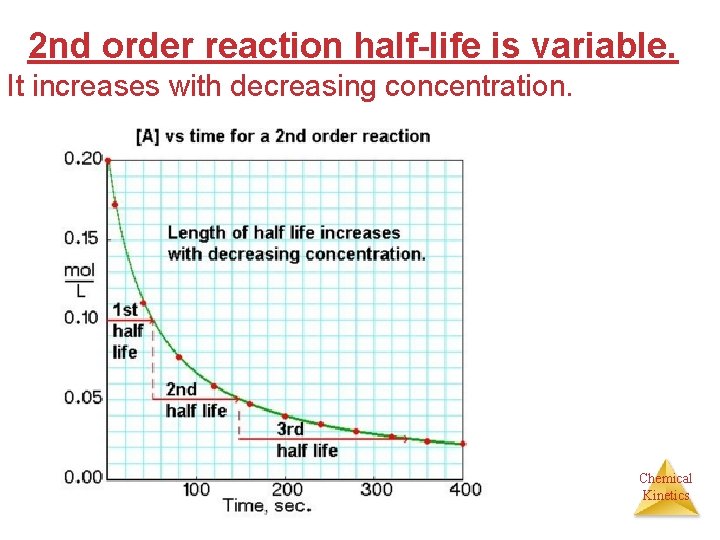
Half-Life for a Second- Order Reaction A A+B products Chemical Kinetics

2 nd order reaction half-life is variable. It increases with decreasing concentration. Chemical Kinetics

Chemical Kinetics

Finding the Overall Rate Law by Utilizing Reaction Mechanisms Chemical Kinetics

Reaction Mechanisms • Not all reactions occur in one step. • The step by step sequence of 2 or more simple reactions that combine to form the overall reaction. • A chemical mechanism describes in detail exactly what takes place at each stage of an overall chemical reaction. Chemical Kinetics

Reaction Mechanism Terms Complex reaction- overall reaction elementary steps- series of simpler reactions that combine to form the complex reaction. Intermediate-a substance produced in one elementary step and consumed in another. Catalyst-increases the rate but not consumed by the reaction. It remains unchanged. Chemical Kinetics

Multistep Mechanisms • In a multistep process, one of the steps will be slower than all others. • The slowest step is called the ratedetermining step. It will be the one used to find the rate law for the overall reaction. • The overall reaction cannot occur faster than this slowest, rate-determining step Chemical Kinetics

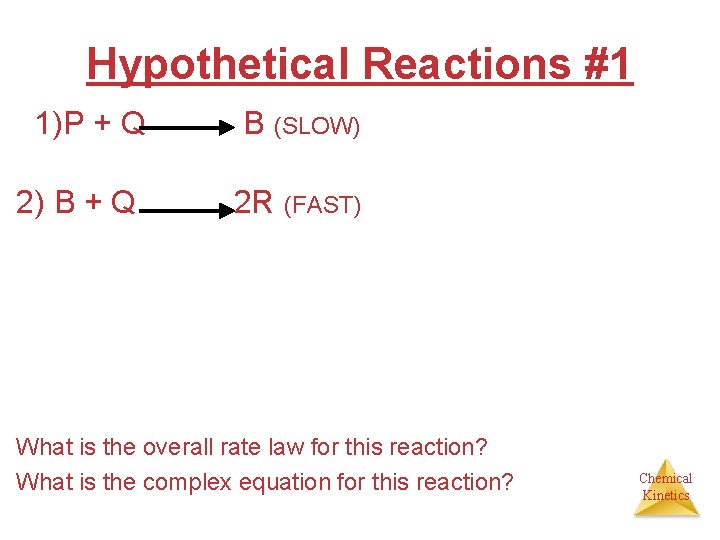
Hypothetical Reactions #1 1)P + Q B (SLOW) 2) B + Q 2 R (FAST) What is the overall rate law for this reaction? What is the complex equation for this reaction? Chemical Kinetics

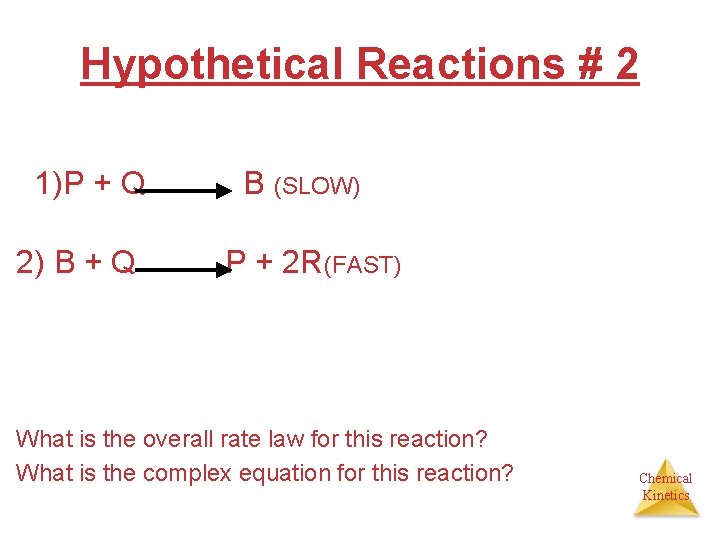
Hypothetical Reactions # 2 1)P + Q 2) B + Q B (SLOW) P + 2 R(FAST) What is the overall rate law for this reaction? What is the complex equation for this reaction? Chemical Kinetics

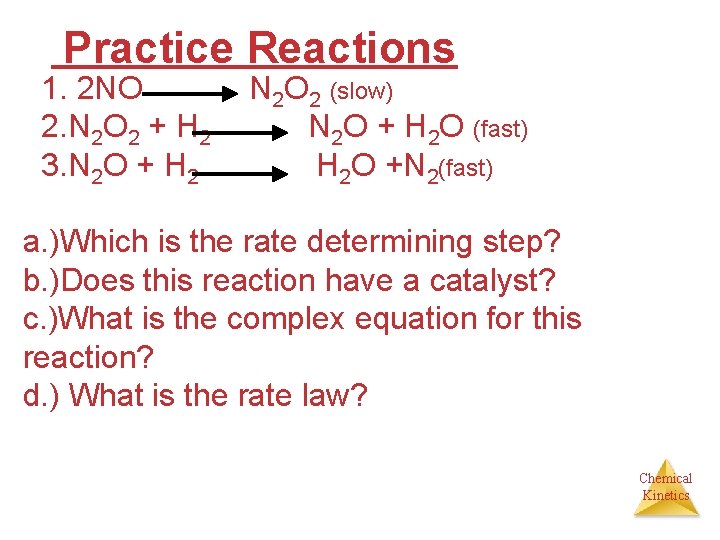
Practice Reactions 1. 2 NO 2. N 2 O 2 + H 2 3. N 2 O + H 2 N 2 O 2 (slow) N 2 O + H 2 O (fast) H 2 O +N 2(fast) a. )Which is the rate determining step? b. )Does this reaction have a catalyst? c. )What is the complex equation for this reaction? d. ) What is the rate law? Chemical Kinetics

Answers to practice problem A. ) Step one is the rate determining step B. ) No catalyst C. ) 2 NO + 2 H 2 N 2 + 2 H 2 O D. ) rate = k [NO]2 Chemical Kinetics

Please Remember!!!! • YOU CAN ONLY USE THE COEFFICIENT METHOD TO DETERMINE THE RATE LAW OF ELEMENTARY STEPS. • YOU CANNOT USE THE COEFFICIENTS FROM THE COMPLEX EQUATION AND DO THE SAME THING. Chemical Kinetics
 Molecularity of reaction
Molecularity of reaction Chemistry grade 11 unit 4
Chemistry grade 11 unit 4 Kinetics order
Kinetics order Chemical kinetics definition
Chemical kinetics definition Chemical kinetics experiment
Chemical kinetics experiment Applications of chemical kinetics
Applications of chemical kinetics Steady state approximation example
Steady state approximation example Paradigm shift from women studies to gender studies
Paradigm shift from women studies to gender studies Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Chụp phim tư thế worms-breton
Chụp phim tư thế worms-breton Chúa yêu trần thế
Chúa yêu trần thế Kể tên các môn thể thao
Kể tên các môn thể thao Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Cong thức tính động năng
Cong thức tính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế
Cái miệng nó xinh thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Giọng cùng tên là
Giọng cùng tên là Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dot
Dot Bảng số nguyên tố lớn hơn 1000
Bảng số nguyên tố lớn hơn 1000 Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Hươu thường đẻ mỗi lứa mấy con
Hươu thường đẻ mỗi lứa mấy con Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Hệ hô hấp
Hệ hô hấp Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu