Advanced Stoichiometry Chapter 9 Section 2 pages 312



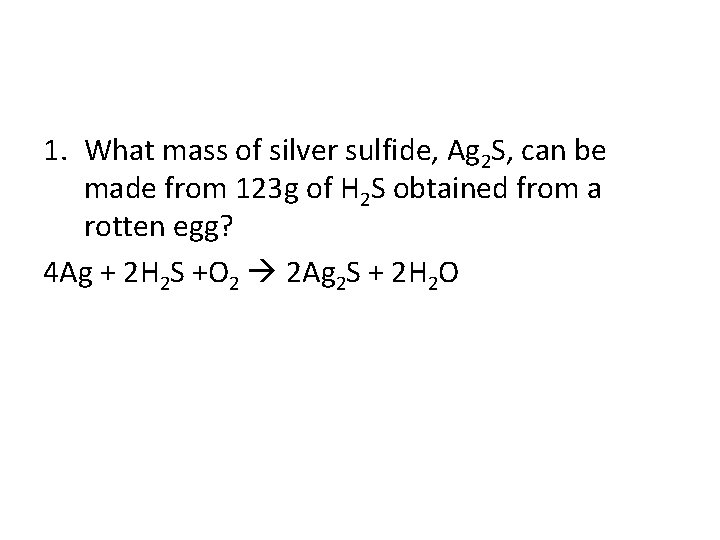
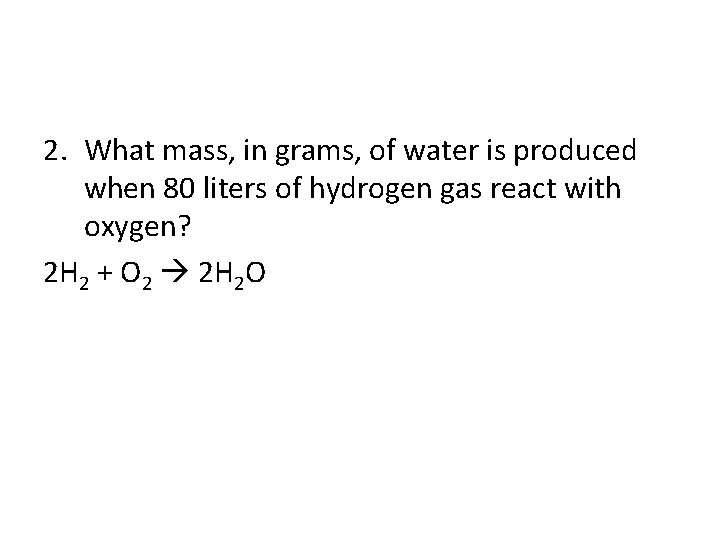

- Slides: 14

Advanced Stoichiometry Chapter 9, Section 2 (pages 312 – 318) Problem Set E (p. 314 # 1 – 3) Problem Set F (p. 317 # 1 – 3)

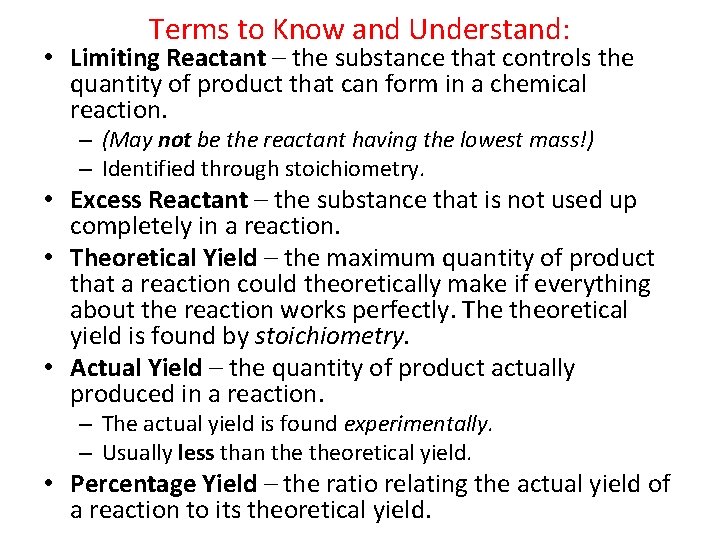
Terms to Know and Understand: • Limiting Reactant – the substance that controls the quantity of product that can form in a chemical reaction. – (May not be the reactant having the lowest mass!) – Identified through stoichiometry. • Excess Reactant – the substance that is not used up completely in a reaction. • Theoretical Yield – the maximum quantity of product that a reaction could theoretically make if everything about the reaction works perfectly. The theoretical yield is found by stoichiometry. • Actual Yield – the quantity of product actually produced in a reaction. – The actual yield is found experimentally. – Usually less than theoretical yield. • Percentage Yield – the ratio relating the actual yield of a reaction to its theoretical yield.

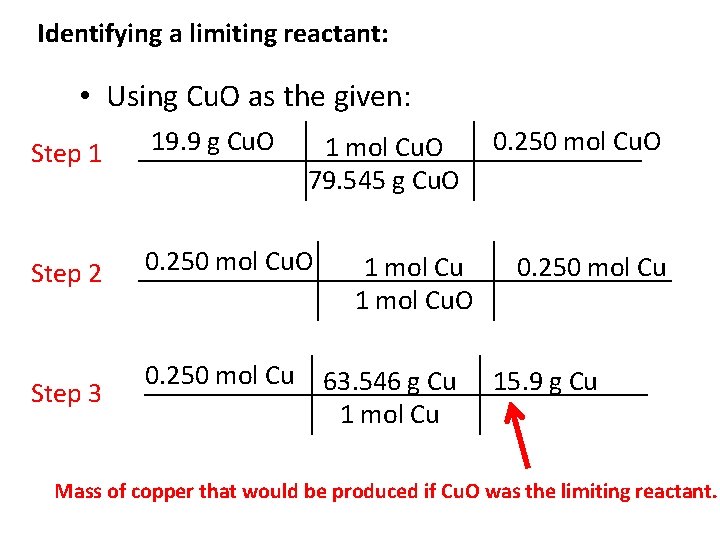
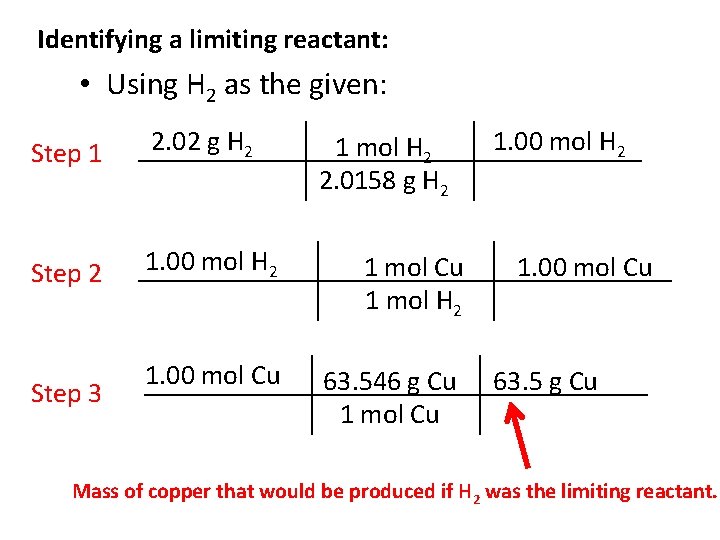
Identifying a limiting reactant: Example: A chemical reaction occurs between copper (II) oxide and hydrogen according to the following balanced equation: Cu. O (s) + H 2 (g) Cu (s) + H 2 O (g) (a) What is the limiting reactant when 19. 9 -g of Cu. O react with 2. 02 -g H 2? (b) What is theoretical yield of copper? • First read through other questions in the problem to see if you will be asked to find theoretical yield of one of the products – if this is the case, do stoichiometry using this product as your ‘find’. • If you are not asked to find the mass of one of the products, choose one as your ‘find’. • Do the stoichiometry twice using each reactant as your ‘given’. To do the remaining work I will use Cu (s) as my find’.

Identifying a limiting reactant: • Using Cu. O as the given: Step 1 19. 9 g Cu. O Step 2 0. 250 mol Cu. O Step 3 0. 250 mol Cu 1 mol Cu. O 79. 545 g Cu. O 1 mol Cu. O 63. 546 g Cu 1 mol Cu 0. 250 mol Cu. O 0. 250 mol Cu 15. 9 g Cu Mass of copper that would be produced if Cu. O was the limiting reactant.

Identifying a limiting reactant: • Using H 2 as the given: Step 1 2. 02 g H 2 Step 2 1. 00 mol H 2 1 mol Cu 1 mol H 2 1. 00 mol Cu 63. 546 g Cu 1 mol Cu Step 3 1 mol H 2 2. 0158 g H 2 1. 00 mol Cu 63. 5 g Cu Mass of copper that would be produced if H 2 was the limiting reactant.

Identifying a limiting reactant: • Compare the calculated quantities of product: – When 15. 9 -g of Cu are produced, the 19. 9 -g of Cu. O will be completely consumed (used up). – If 63. 5 -g of Cu were produced, 2. 02 g of H 2 would be completely consumed, but there is not enough Cu. O to do this. • The limiting reactant is Cu. O, because it will be gone after 15. 9 -g of Cu is produced, and the reaction will stop. • The excess reactant is H 2, because the amount available would allow the reaction to produce 63. 5 -g Cu, but the reaction stops when the limiting reactant is used up. • The theoretical yield of Cu will be 15. 9 -g, because the reaction will stop as soon as this amount of product is formed.

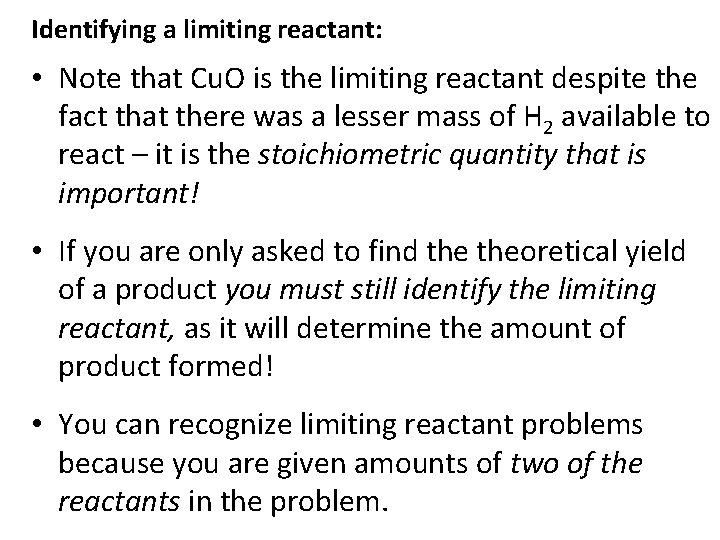
Identifying a limiting reactant: • Note that Cu. O is the limiting reactant despite the fact that there was a lesser mass of H 2 available to react – it is the stoichiometric quantity that is important! • If you are only asked to find theoretical yield of a product you must still identify the limiting reactant, as it will determine the amount of product formed! • You can recognize limiting reactant problems because you are given amounts of two of the reactants in the problem.

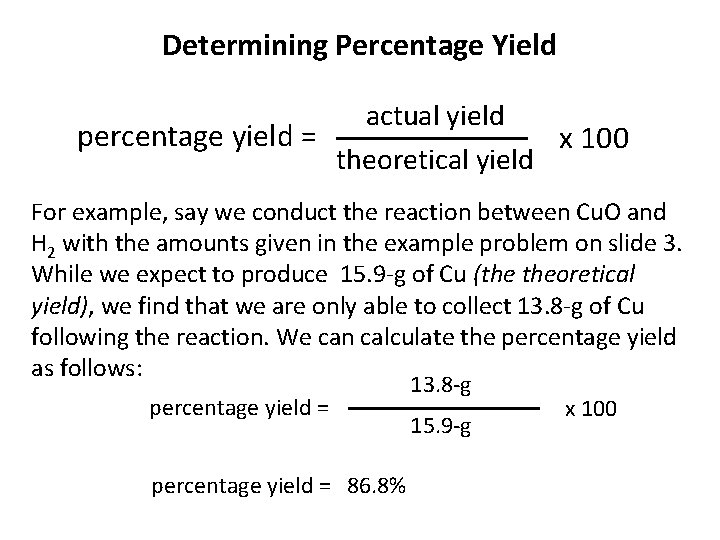
Determining Percentage Yield actual yield percentage yield = x 100 theoretical yield For example, say we conduct the reaction between Cu. O and H 2 with the amounts given in the example problem on slide 3. While we expect to produce 15. 9 -g of Cu (the theoretical yield), we find that we are only able to collect 13. 8 -g of Cu following the reaction. We can calculate the percentage yield as follows: percentage yield = 13. 8 -g 15. 9 -g percentage yield = 86. 8% x 100
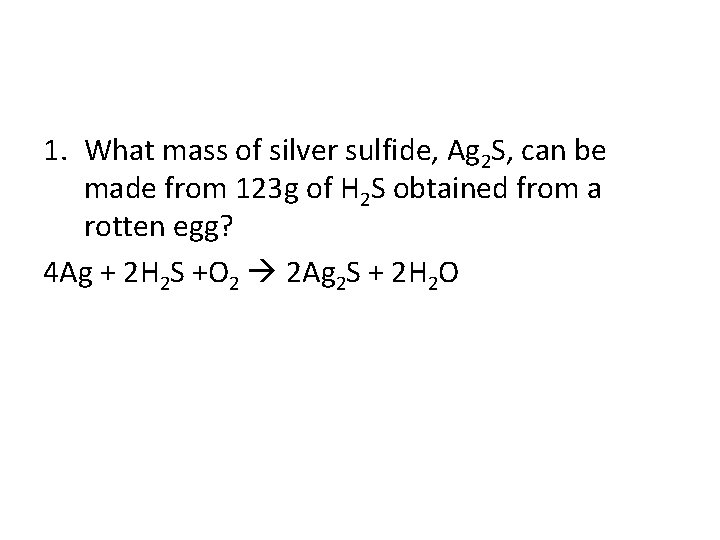
1. What mass of silver sulfide, Ag 2 S, can be made from 123 g of H 2 S obtained from a rotten egg? 4 Ag + 2 H 2 S +O 2 2 Ag 2 S + 2 H 2 O
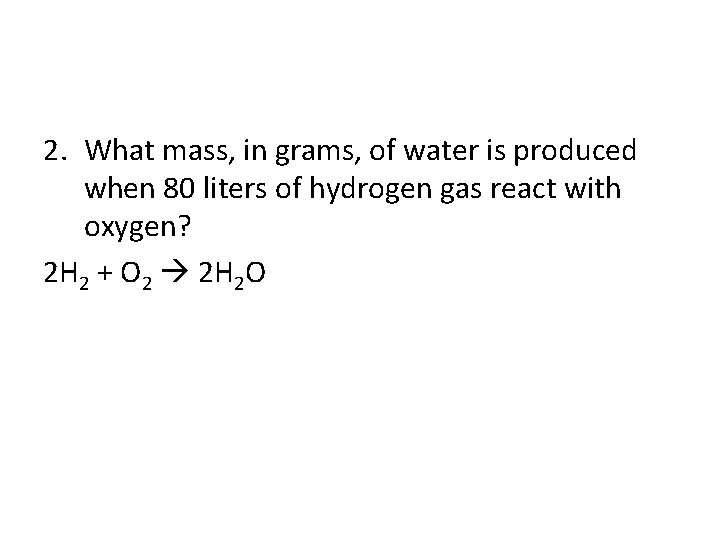
2. What mass, in grams, of water is produced when 80 liters of hydrogen gas react with oxygen? 2 H 2 + O 2 2 H 2 O

• • Practice Box E (p. 314 # 1 – 3) • • Chapter 9 Review, p. 331 # 31, 32, 33

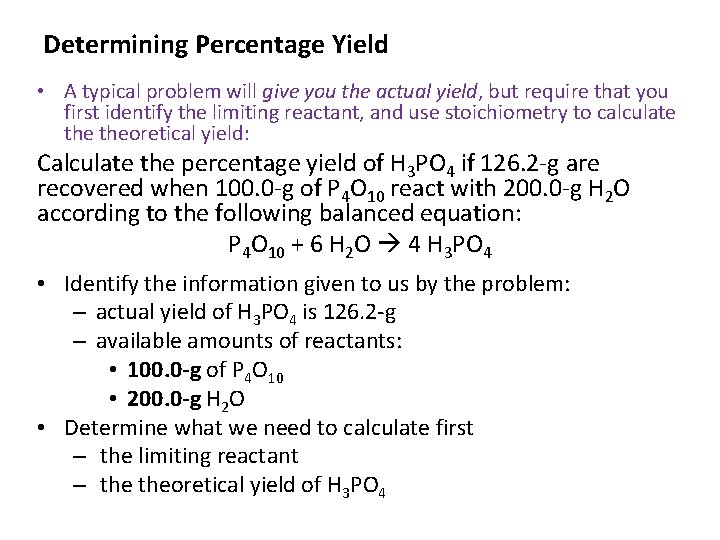
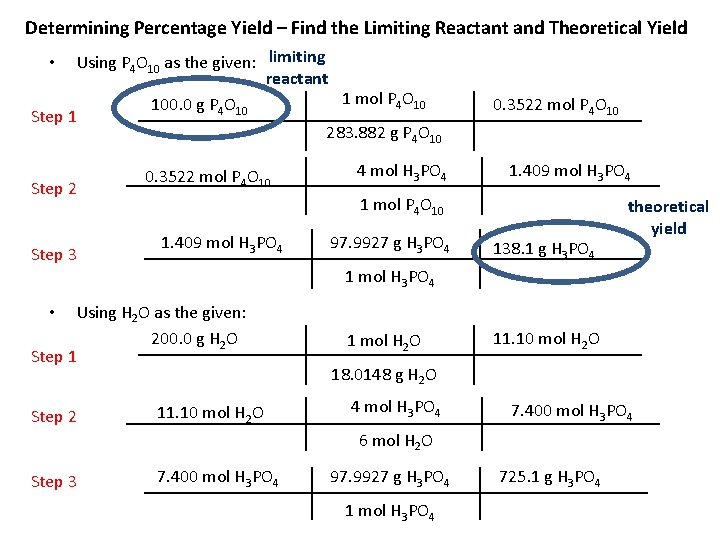
Determining Percentage Yield • A typical problem will give you the actual yield, but require that you first identify the limiting reactant, and use stoichiometry to calculate theoretical yield: Calculate the percentage yield of H 3 PO 4 if 126. 2 -g are recovered when 100. 0 -g of P 4 O 10 react with 200. 0 -g H 2 O according to the following balanced equation: P 4 O 10 + 6 H 2 O 4 H 3 PO 4 • Identify the information given to us by the problem: – actual yield of H 3 PO 4 is 126. 2 -g – available amounts of reactants: • 100. 0 -g of P 4 O 10 • 200. 0 -g H 2 O • Determine what we need to calculate first – the limiting reactant – theoretical yield of H 3 PO 4

Determining Percentage Yield – Find the Limiting Reactant and Theoretical Yield Using P 4 O 10 as the given: limiting reactant 1 mol P 4 O 10 100. 0 g P 4 O 10 Step 1 283. 882 g P 4 O 10 • Step 2 Step 3 0. 3522 mol P 4 O 10 4 mol H 3 PO 4 0. 3522 mol P 4 O 10 1. 409 mol H 3 PO 4 1 mol P 4 O 10 1. 409 mol H 3 PO 4 97. 9927 g H 3 PO 4 138. 1 g H 3 PO 4 theoretical yield 1 mol H 3 PO 4 Using H 2 O as the given: 200. 0 g H 2 O Step 1 • Step 2 11. 10 mol H 2 O O 2 O 1 mol H 11. 10 mol H 2 O 18. 0148 g H 2 O 4 mol H 3 PO 4 7. 400 mol H 3 PO 4 6 mol H 2 O Step 3 7. 400 mol H 3 PO 4 97. 9927 g H 3 PO 4 1 mol H 3 PO 4 725. 1 g H 3 PO 4

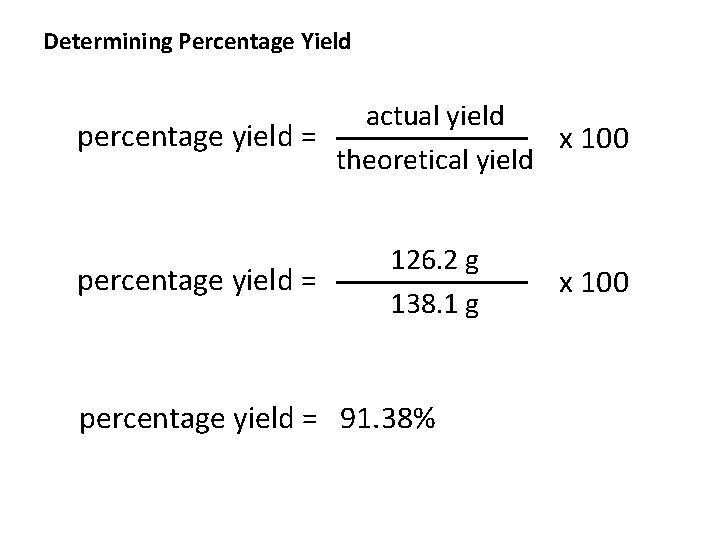
Determining Percentage Yield actual yield percentage yield = x 100 theoretical yield percentage yield = 126. 2 g 138. 1 g percentage yield = 91. 38% x 100