Chapter 12 Stoichiometry Stoichiometry n Stoichiometry The calculations







- Slides: 16

Chapter 12: Stoichiometry


Stoichiometry n Stoichiometry – The calculations of quantities of substances involved in chemical reactions.

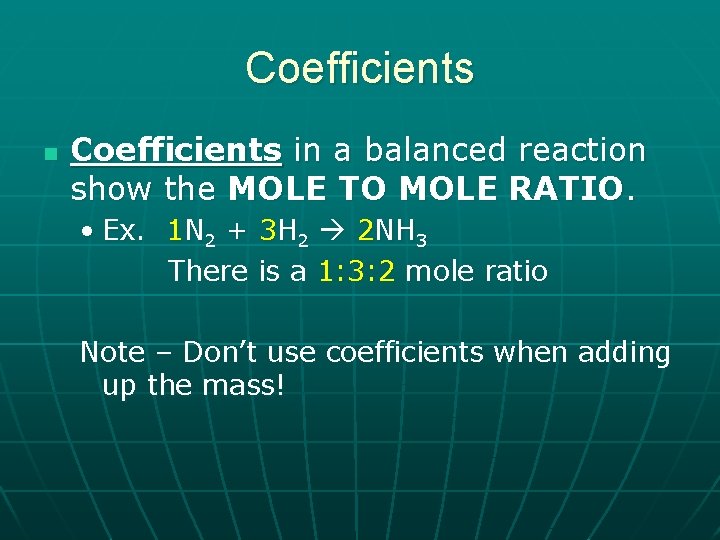
Coefficients n Coefficients in a balanced reaction show the MOLE TO MOLE RATIO. • Ex. 1 N 2 + 3 H 2 2 NH 3 There is a 1: 3: 2 mole ratio Note – Don’t use coefficients when adding up the mass!

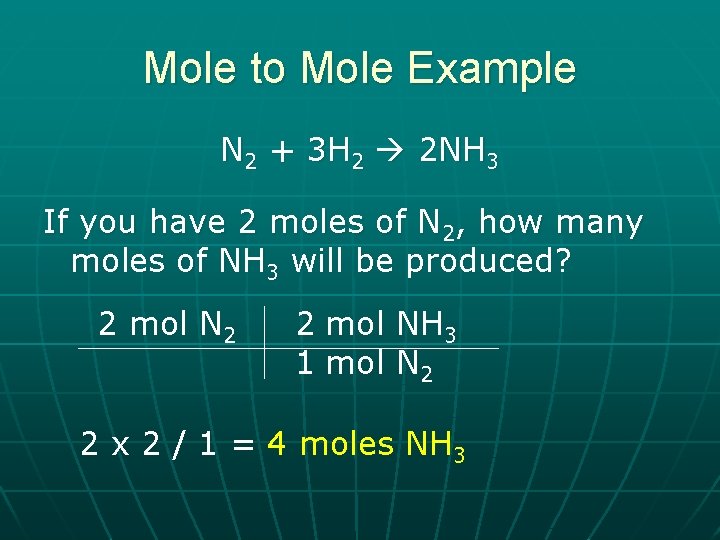
Mole to Mole Example N 2 + 3 H 2 2 NH 3 If you have 2 moles of N 2, how many moles of NH 3 will be produced? 2 mol N 2 2 mol NH 3 1 mol N 2 2 x 2 / 1 = 4 moles NH 3

Mole to Mole Example N 2 + 3 H 2 2 NH 3 If you want 5 moles of product, how many moles of hydrogen gas do you need? 5 mol NH 3 3 mol H 2 2 mol NH 3 5 x 3 / 2 = 7. 5 moles H 2

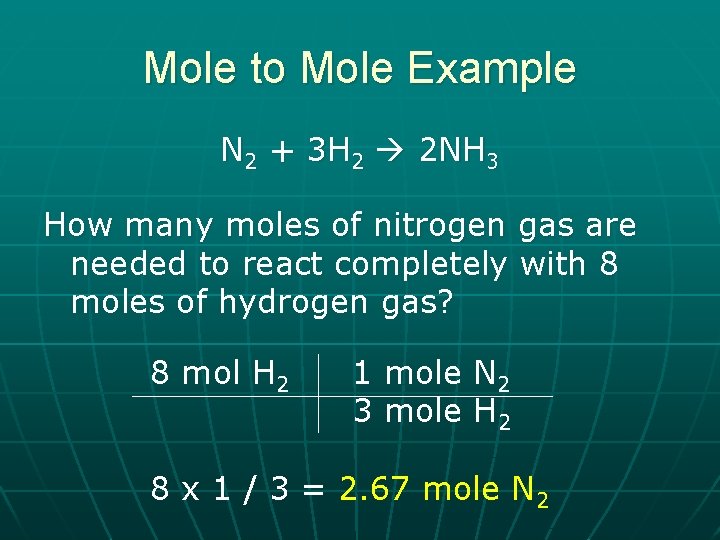
Mole to Mole Example N 2 + 3 H 2 2 NH 3 How many moles of nitrogen gas are needed to react completely with 8 moles of hydrogen gas? 8 mol H 2 1 mole N 2 3 mole H 2 8 x 1 / 3 = 2. 67 mole N 2

Reaction Conversions ****The only way to convert from one compound to something totally different in the reaction is to use the MOLE TO MOLE RATIO from the coefficients!!!****

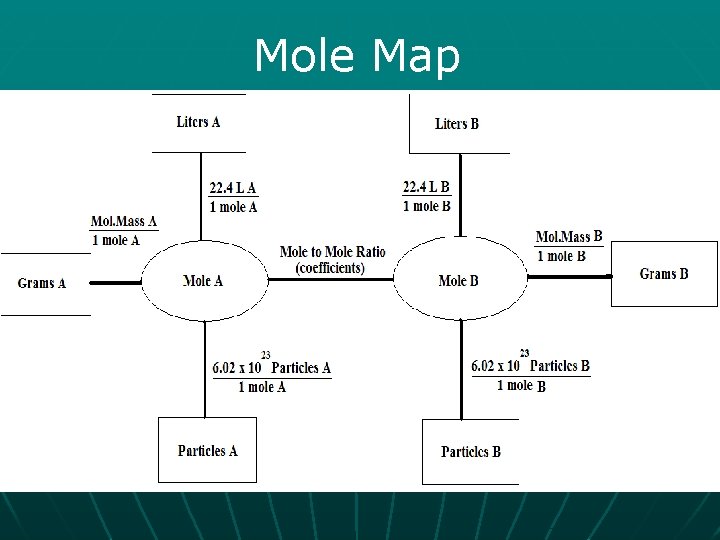
Reaction Conversions Note – If you don’t have moles already, your first step is to convert to moles! 22. 4 L at STP Mole Review: 1 mole Molar Mass 6. 02 x 1023 particles

Mole Map

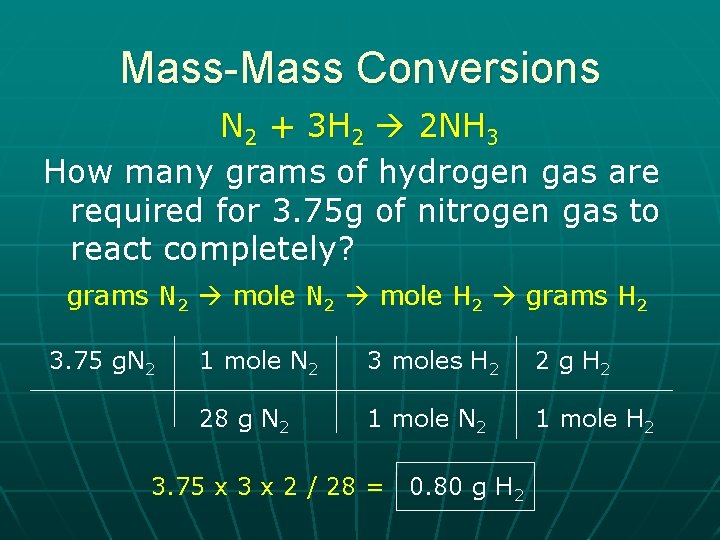
Mass-Mass Conversions N 2 + 3 H 2 2 NH 3 How many grams of hydrogen gas are required for 3. 75 g of nitrogen gas to react completely? grams N 2 mole H 2 grams H 2 3. 75 g. N 2 1 mole N 2 3 moles H 2 2 g H 2 28 g N 2 1 mole H 2 3. 75 x 3 x 2 / 28 = 0. 80 g H 2

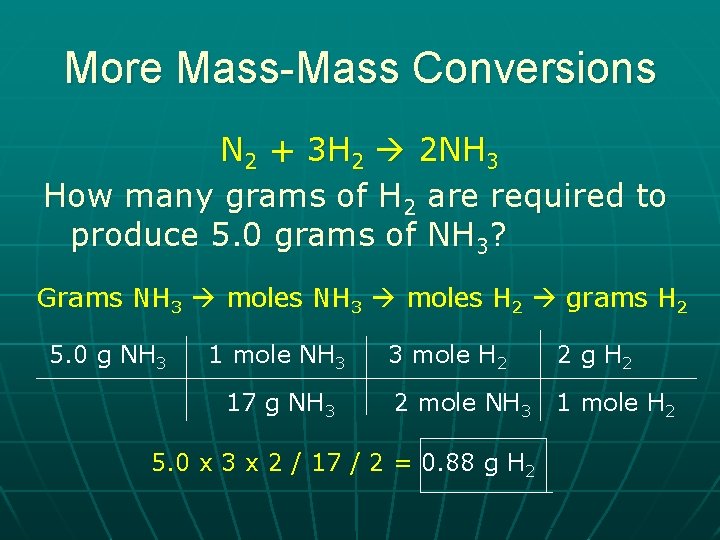
More Mass-Mass Conversions N 2 + 3 H 2 2 NH 3 How many grams of H 2 are required to produce 5. 0 grams of NH 3? Grams NH 3 moles H 2 grams H 2 5. 0 g NH 3 1 mole NH 3 17 g NH 3 3 mole H 2 2 g H 2 2 mole NH 3 1 mole H 2 5. 0 x 3 x 2 / 17 / 2 = 0. 88 g H 2

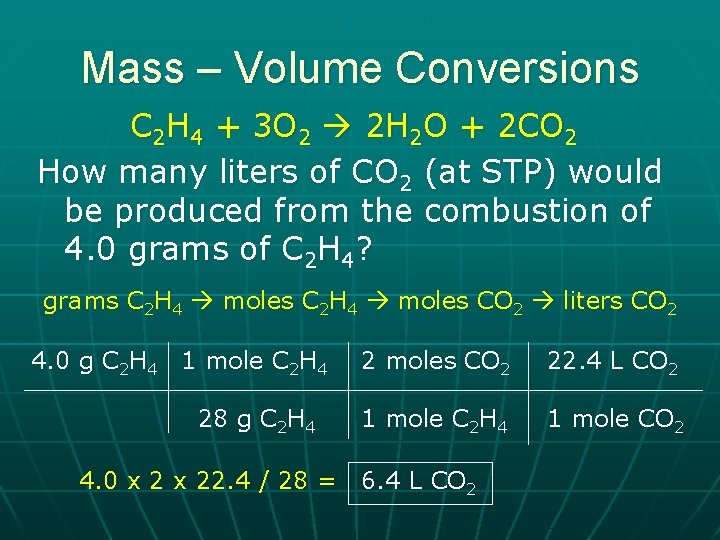
Mass – Volume Conversions C 2 H 4 + 3 O 2 2 H 2 O + 2 CO 2 How many liters of CO 2 (at STP) would be produced from the combustion of 4. 0 grams of C 2 H 4? grams C 2 H 4 moles CO 2 liters CO 2 4. 0 g C 2 H 4 1 mole C 2 H 4 2 moles CO 2 22. 4 L CO 2 28 g C 2 H 4 1 mole CO 2 4. 0 x 22. 4 / 28 = 6. 4 L CO 2

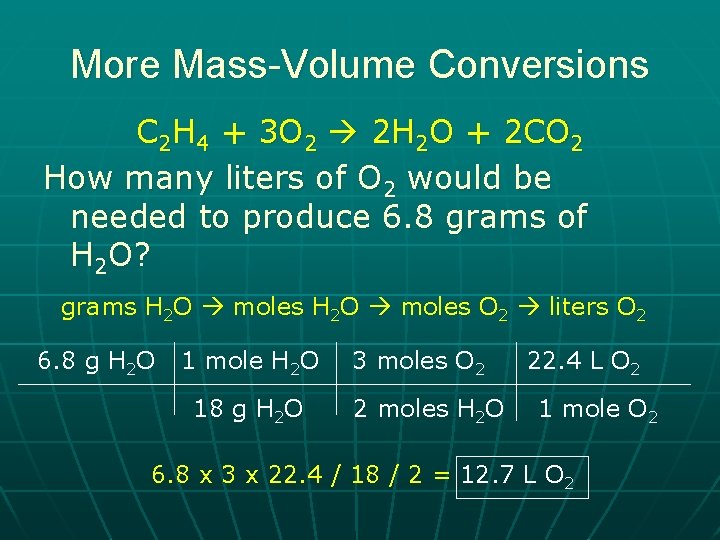
More Mass-Volume Conversions C 2 H 4 + 3 O 2 2 H 2 O + 2 CO 2 How many liters of O 2 would be needed to produce 6. 8 grams of H 2 O? grams H 2 O moles O 2 liters O 2 6. 8 g H 2 O 1 mole H 2 O 18 g H 2 O 3 moles O 2 2 moles H 2 O 22. 4 L O 2 1 mole O 2 6. 8 x 3 x 22. 4 / 18 / 2 = 12. 7 L O 2

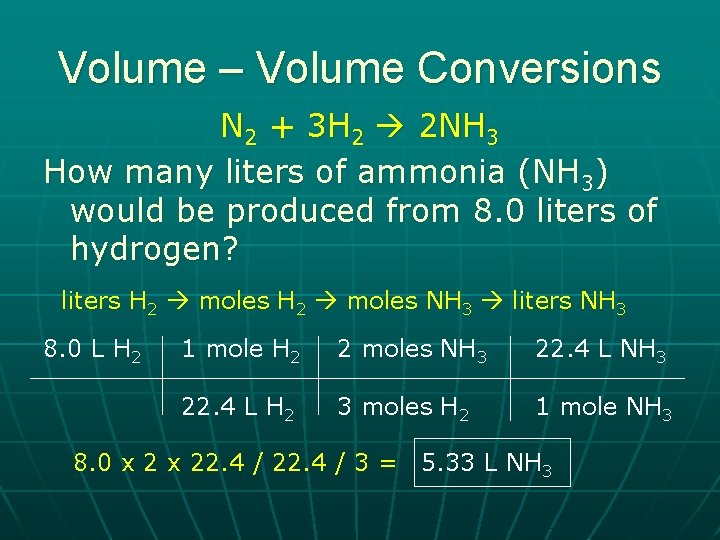
Volume – Volume Conversions N 2 + 3 H 2 2 NH 3 How many liters of ammonia (NH 3) would be produced from 8. 0 liters of hydrogen? liters H 2 moles NH 3 liters NH 3 8. 0 L H 2 1 mole H 2 2 moles NH 3 22. 4 L H 2 3 moles H 2 1 mole NH 3 8. 0 x 22. 4 / 3 = 5. 33 L NH 3

More Volume-Volume Conversions N 2 + 3 H 2 2 NH 3 How many liters of N 2 would be needed to produce 15. 0 liters of NH 3? liters NH 3 moles N 2 liters N 2 15. 0 L NH 3 1 mole NH 3 22. 4 L NH 3 1 mole N 2 2 moles NH 3 15 x 22. 4 / 2 = 7. 5 L N 2 22. 4 L N 2 1 mole N 2