Stoichiometry Chapter 9 Stoichiometry 1 Stoichiometry stoicheion element




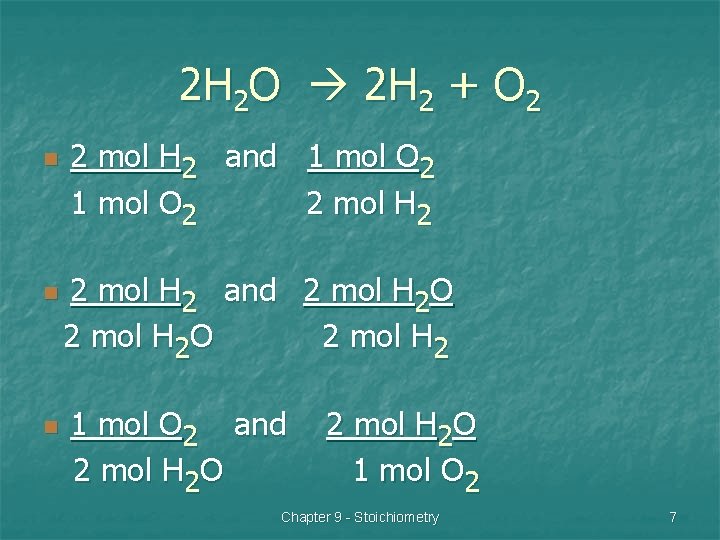



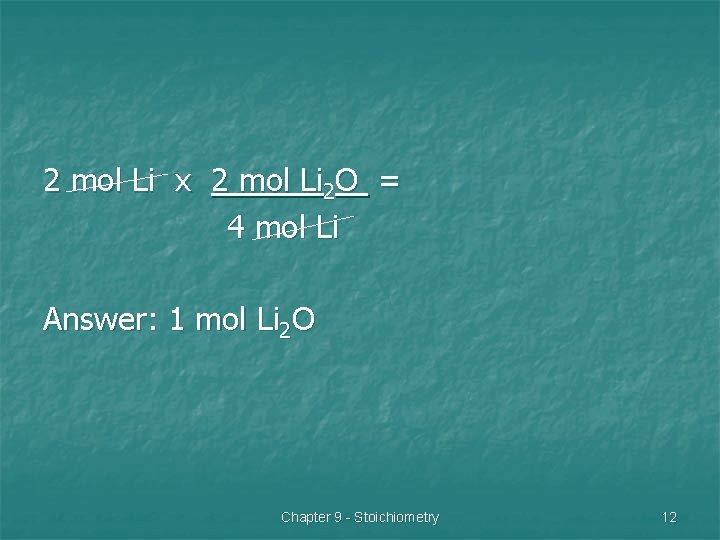








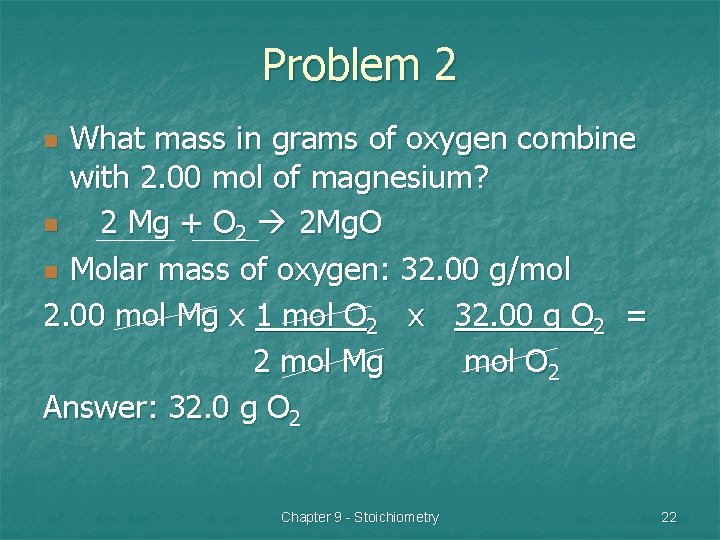











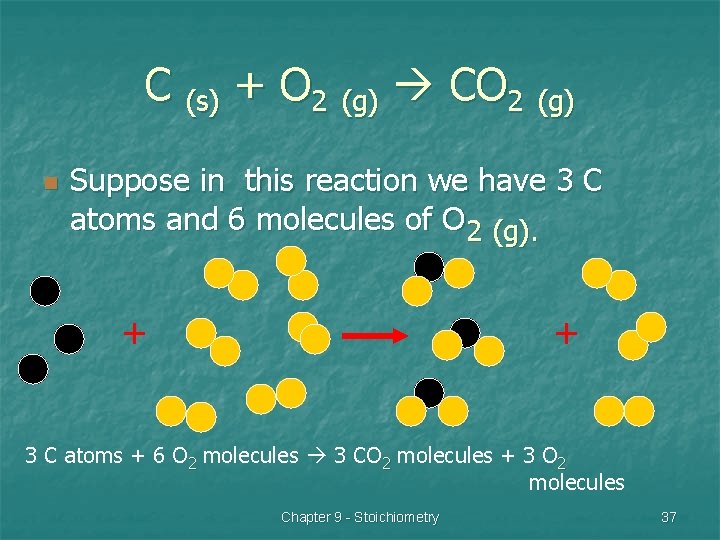







- Slides: 51

Stoichiometry Chapter 9 - Stoichiometry 1

Stoichiometry stoicheion: element metron: measure Chapter 9 - Stoichiometry 2

Reaction Stoichiometry n The study of the mass relationships between reactants and products in a chemical reaction. Chapter 9 - Stoichiometry 3

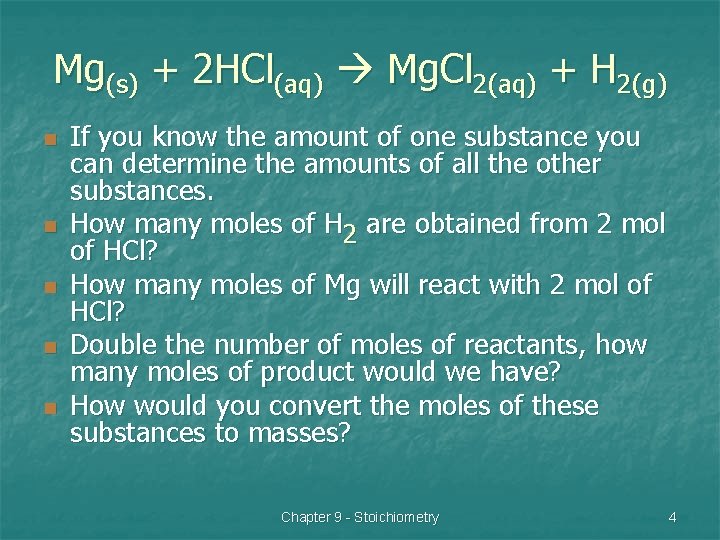
Mg(s) + 2 HCl(aq) Mg. Cl 2(aq) + H 2(g) n n n If you know the amount of one substance you can determine the amounts of all the other substances. How many moles of H 2 are obtained from 2 mol of HCl? How many moles of Mg will react with 2 mol of HCl? Double the number of moles of reactants, how many moles of product would we have? How would you convert the moles of these substances to masses? Chapter 9 - Stoichiometry 4

To solve stoichiometry problems you must have: n A balanced chemical equation to determine the correct mole ratios Chapter 9 - Stoichiometry 5

Mole Ratio n A conversion factor that relates the amounts in moles of any two substances involved in a chemical reaction. Chapter 9 - Stoichiometry 6
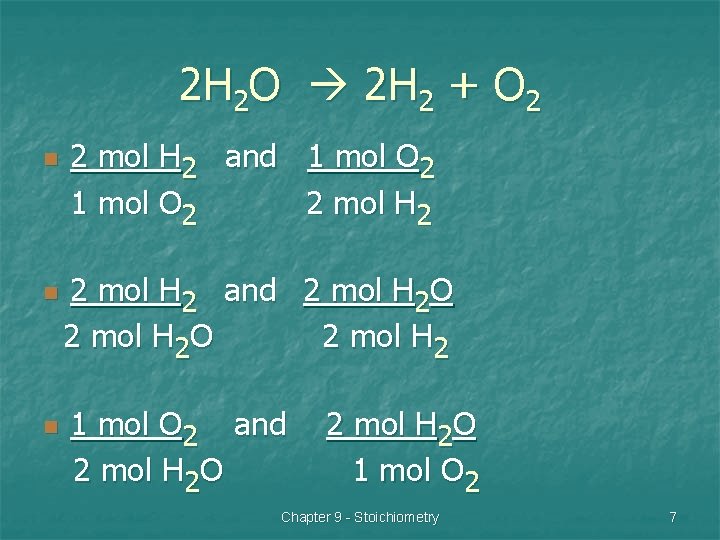
2 H 2 O 2 H 2 + O 2 n n n 2 mol H 2 and 1 mol O 2 2 mol H 2 and 2 mol H 2 O 2 mol H 2 1 mol O 2 and 2 mol H 2 O 1 mol O 2 Chapter 9 - Stoichiometry 7

2 Al 2 O 3 (l) 4 Al (s) + 3 O 2 (g) n What are the possible mole ratios? Chapter 9 - Stoichiometry 8

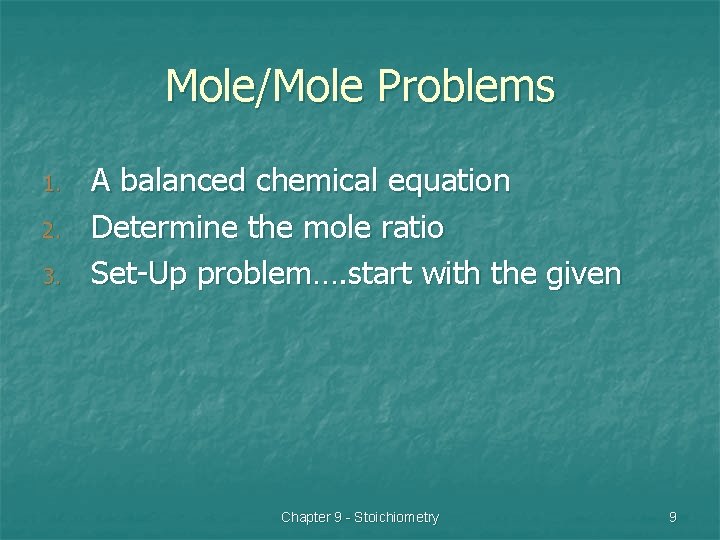
Mole/Mole Problems 1. 2. 3. A balanced chemical equation Determine the mole ratio Set-Up problem…. start with the given Chapter 9 - Stoichiometry 9

Example 1: n The elements lithium and oxygen react to form lithium oxide. How many mol of lithium oxide will form if 2 mol of lithium react? Chapter 9 - Stoichiometry 10

Li + O 2 Li 2 O 1. Balance equation: 4 Li + O 2 2 Li 2 O 2. Mole ratio of lithium to lithium oxide: 4 mol lithium to 2 mol lithium oxide 2. Set-Up problem, start with given. Chapter 9 - Stoichiometry 11
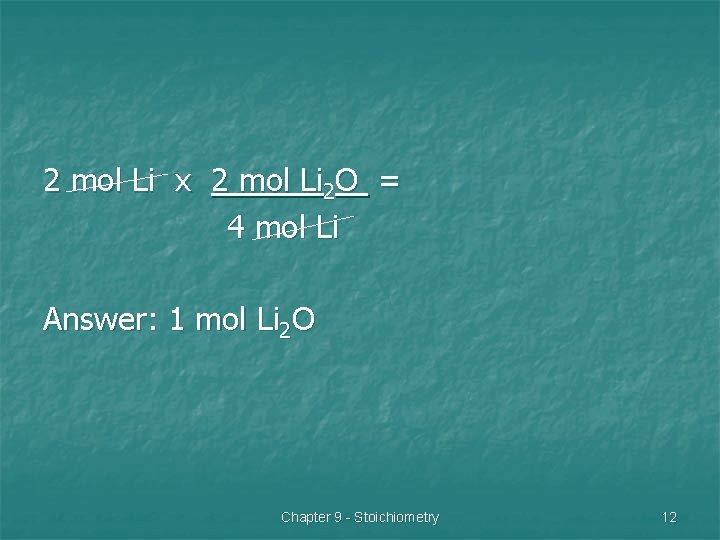
2 mol Li x 2 mol Li 2 O = 4 mol Li Answer: 1 mol Li 2 O Chapter 9 - Stoichiometry 12

Problem 2 n Hydrogen peroxide decomposes to form water and oxygen gas. How many moles of oxygen will result from the decomposition of 5. 0 mol of H 2 O 2? Chapter 9 - Stoichiometry 13

H 2 O 2 H 2 O + O 2 1. 2. 3. Balance equation: 2 H 2 O 2 2 H 2 O + O 2 Mole ratio of oxygen & hydrogen peroxide: 2 mol H 2 O 2 to 1 mol O 2 Set-up problem starting with the given. Chapter 9 - Stoichiometry 14

5. O mol H 2 O 2 x 1 mol O 2 = 2 mol H 2 O 2 Answer: 2. 5 mol of O 2 Chapter 9 - Stoichiometry 15

Problem 3 n Ammonia, NH 3 is widely used as a fertilizer and in many household cleaners. How many moles of ammonia are produced when 6 mol of hydrogen gas react with an excess of nitrogen gas? Chapter 9 - Stoichiometry 16

N 2 + H 2 NH 3 1. 2. 3. Balance equation: N 2 + 3 H 2 2 NH 3 Determine mol ratio of hydrogen to ammonia: 3 mol hydrogen to 2 mol ammonia Set-up problem starting with the given. Chapter 9 - Stoichiometry 17

6 mol H 2 x 2 mol NH 3 = 3 mol H 2 Answer: 4 mol NH 3 Chapter 9 - Stoichiometry 18

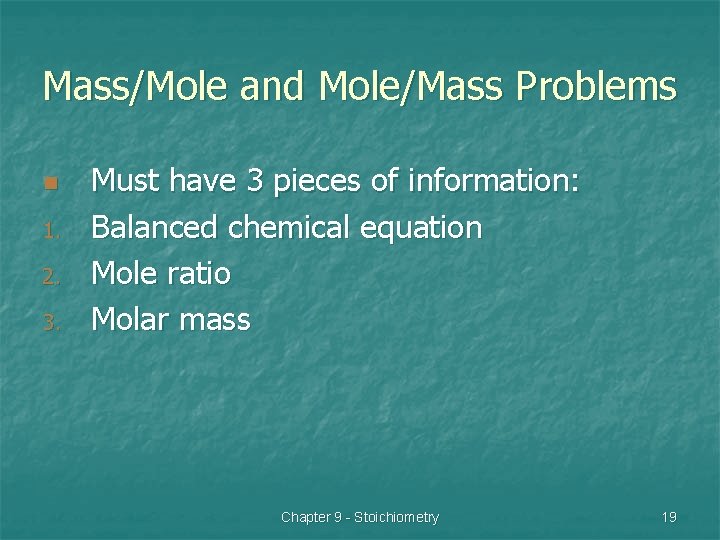
Mass/Mole and Mole/Mass Problems n 1. 2. 3. Must have 3 pieces of information: Balanced chemical equation Mole ratio Molar mass Chapter 9 - Stoichiometry 19

Problem 1 What mass in grams of magnesium oxide is produced from 2. 00 mol of magnesium? n Mg + O 2 Mg. O n 2 Mg + O 2 2 Mg. O n Molar Mass of Mg. O n 40. 31 g/mol 2. 00 mol Mg x 2 mol Mg. O x 40. 31 g Mg. O = 2 mol Mg. O n Chapter 9 - Stoichiometry 20

n Answer: 80. 62 g Mg. O = 80. 6 g Mg. O Chapter 9 - Stoichiometry 21
Problem 2 What mass in grams of oxygen combine with 2. 00 mol of magnesium? n 2 Mg + O 2 2 Mg. O n Molar mass of oxygen: 32. 00 g/mol 2. 00 mol Mg x 1 mol O 2 x 32. 00 g O 2 = 2 mol Mg mol O 2 Answer: 32. 0 g O 2 n Chapter 9 - Stoichiometry 22

Problem 3 n n n How many mol of Hg. O are needed to produce 125 grams of oxygen? Hg. O Hg + O 2 2 Hg. O 2 Hg + O 2 Molar mass of oxygen: 32. 00 g/mol 125 g O 2 x mol O 2 x 2 mol Hg. O = 32. 00 g O 2 1 mol O 2 Answer: 7. 81 mol Hg. O Chapter 9 - Stoichiometry 23

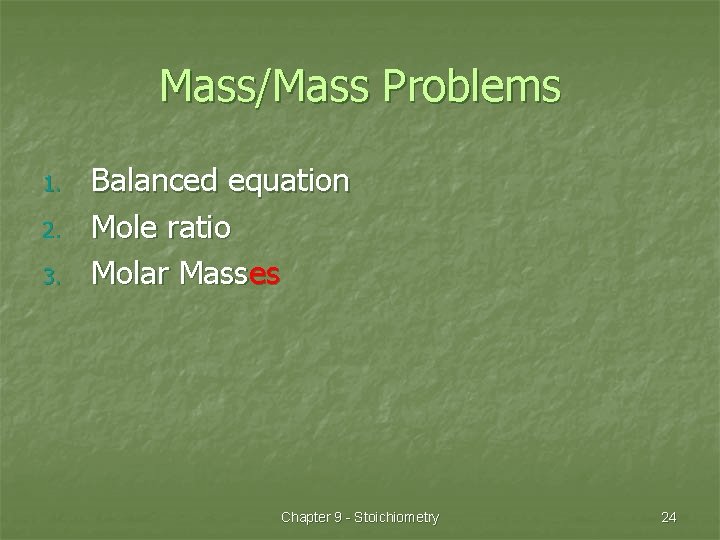
Mass/Mass Problems 1. 2. 3. Balanced equation Mole ratio Molar Masses Chapter 9 - Stoichiometry 24

Problem 1 n n n Tin (II) fluoride, Sn. F 2 is used in toothpaste. How many grams of tin (II) fluoride are produced from the reaction of 30. 0 g of HF with Sn? (Hydrogen is also a product. ) Sn + HF Sn. F 2 + H 2 Sn + 2 HF Sn. F 2 + H 2 Chapter 9 - Stoichiometry 25

Sn + 2 HF Sn. F 2 + H 2 n n n How many grams of tin (II) fluoride are produced from the reaction of 30. 0 g of HF with Sn? Mole ratio? 2 mole HF : 1 mole Sn. F 2 Molar masses of HF and Sn. F 2? HF: 20. 01 g/mol Sn. F 2: 156. 71 g/mol Chapter 9 - Stoichiometry 26

How many grams of Sn. F 2 are produced from the reaction of 30. 0 g of HF with Sn? 30. 0 g. HF x mol HF x 1 mol Sn. F 2 x 156. 71 g. Sn. F 2 20. 01 g. HF 2 mol HF mol Sn. F 2 Answer: 117 g Sn. F 2 Chapter 9 - Stoichiometry 27

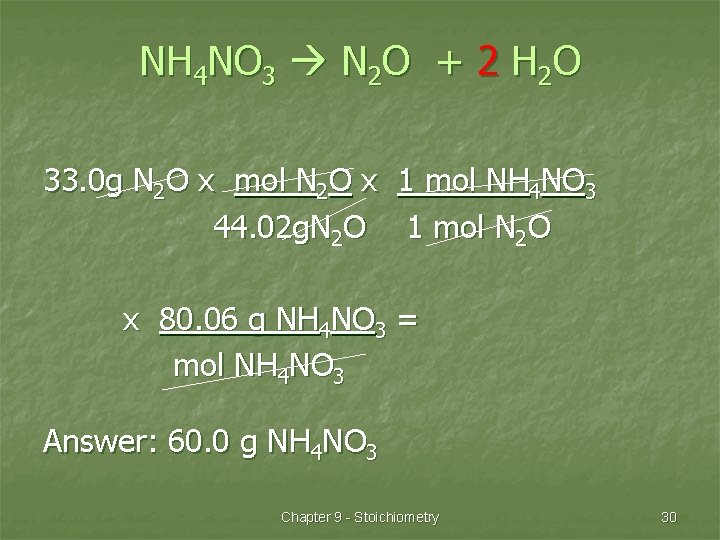
Problem 2 n n n Nitrous oxide, N 2 O (nitrogen I oxide) a. k. a. laughing gas is used as an anesthetic in dentistry. How many grams of NH 4 NO 3 is required to form 33. 0 g N 2 O? (the other product of this decomposition reaction is water) NH 4 NO 3 N 2 O + H 2 O NH 4 NO 3 N 2 O + 2 H 2 O Chapter 9 - Stoichiometry 28

NH 4 NO 3 N 2 O + 2 H 2 O n n n Mole Ratio? 1 mol NH 4 NO 3: 1 mol N 2 O Molar Mass of NH 4 NO 3 80. 06 g/mol Molar Mass of N 2 O 44. 02 g/mol Chapter 9 - Stoichiometry 29

NH 4 NO 3 N 2 O + 2 H 2 O 33. 0 g N 2 O x mol N 2 O x 1 mol NH 4 NO 3 44. 02 g. N 2 O 1 mol N 2 O x 80. 06 g NH 4 NO 3 = mol NH 4 NO 3 Answer: 60. 0 g NH 4 NO 3 Chapter 9 - Stoichiometry 30

Stoichiometry 9 -3 Chapter 9 - Stoichiometry 31

Limiting & Excess Reactants n n Limiting Reactant: the reactant is used up first in a chemical reaction. Excess Reactant: the reactant that is not totally used up in a chemical reaction. Chapter 9 - Stoichiometry 32

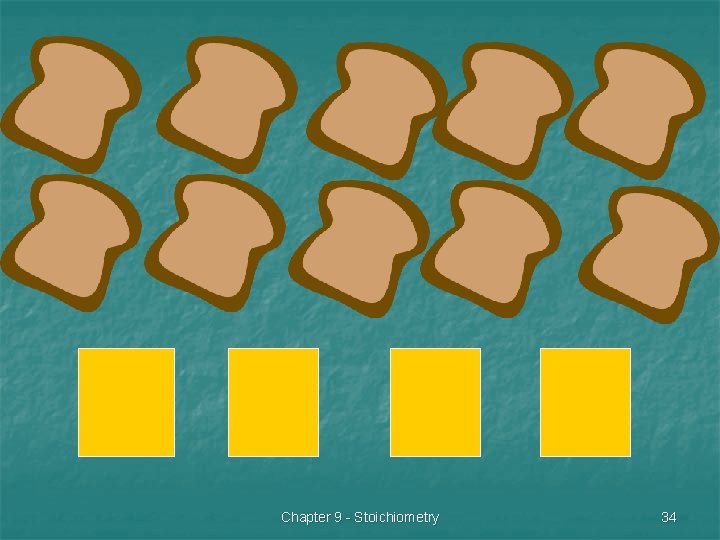
Identify the limiting & excess reactants n Suppose you have 10 slices of bread and four slices of cheese. How many sandwiches can you make? (Assume that the cheese is thick sliced) Chapter 9 - Stoichiometry 33

Chapter 9 - Stoichiometry 34

n n What is the limiting reactant? What is the excess reactant? Chapter 9 - Stoichiometry 35

Musical Chairs n In the game Musical Chairs, what is the limiting reagent and what is the excess reagent? Chapter 9 - Stoichiometry 36
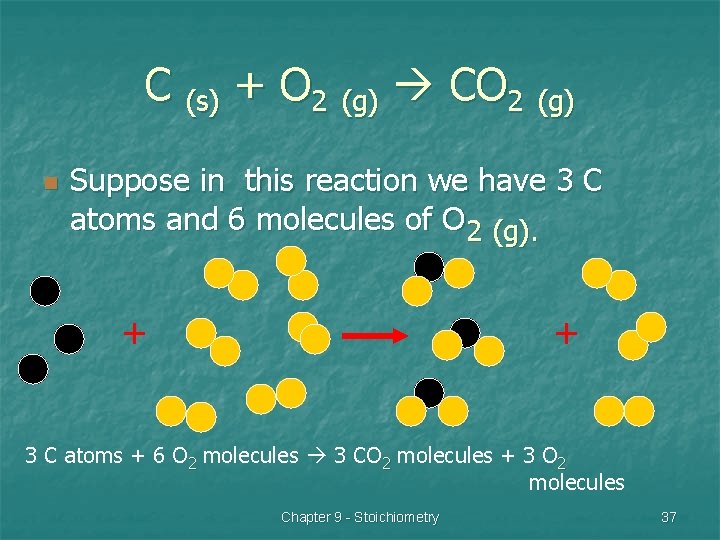
C (s) + O 2 (g) CO 2 (g) n Suppose in this reaction we have 3 C atoms and 6 molecules of O 2 (g). + + 3 C atoms + 6 O 2 molecules 3 CO 2 molecules + 3 O 2 molecules Chapter 9 - Stoichiometry 37

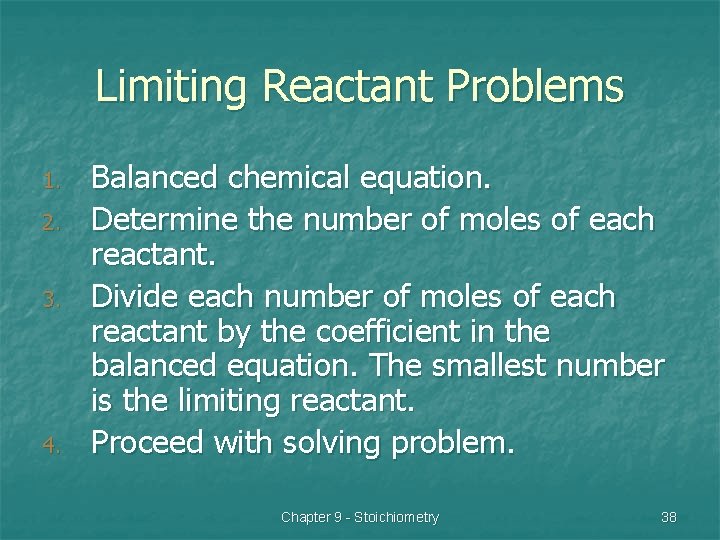
Limiting Reactant Problems 1. 2. 3. 4. Balanced chemical equation. Determine the number of moles of each reactant. Divide each number of moles of each reactant by the coefficient in the balanced equation. The smallest number is the limiting reactant. Proceed with solving problem. Chapter 9 - Stoichiometry 38

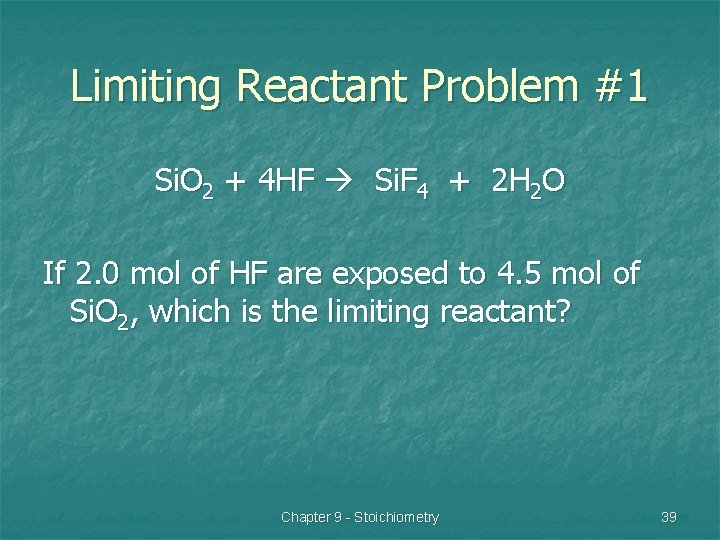
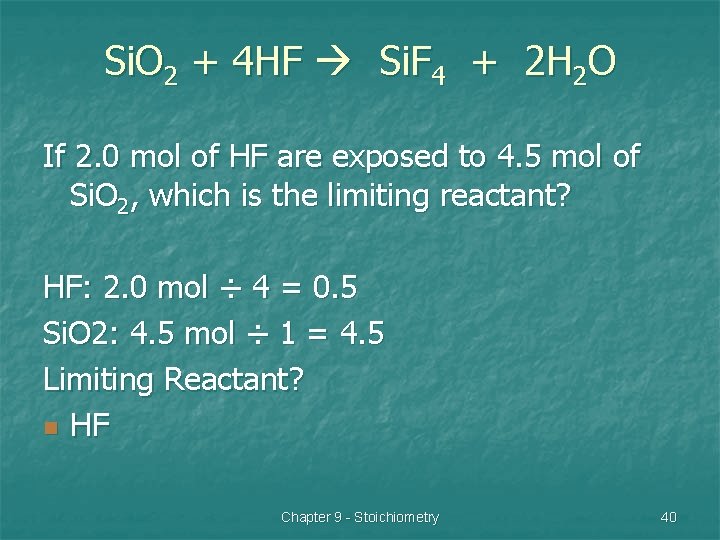
Limiting Reactant Problem #1 Si. O 2 + 4 HF Si. F 4 + 2 H 2 O If 2. 0 mol of HF are exposed to 4. 5 mol of Si. O 2, which is the limiting reactant? Chapter 9 - Stoichiometry 39

Si. O 2 + 4 HF Si. F 4 + 2 H 2 O If 2. 0 mol of HF are exposed to 4. 5 mol of Si. O 2, which is the limiting reactant? HF: 2. 0 mol ÷ 4 = 0. 5 Si. O 2: 4. 5 mol ÷ 1 = 4. 5 Limiting Reactant? n HF Chapter 9 - Stoichiometry 40

Limiting Reactant Problem #2 If 20. 5 g of chlorine is reacted with 20. 5 g of sodium, which is the limiting reactant? How much salt is formed? n n Cl 2 + Na Na. Cl Cl 2 + 2 Na. Cl Chapter 9 - Stoichiometry 41

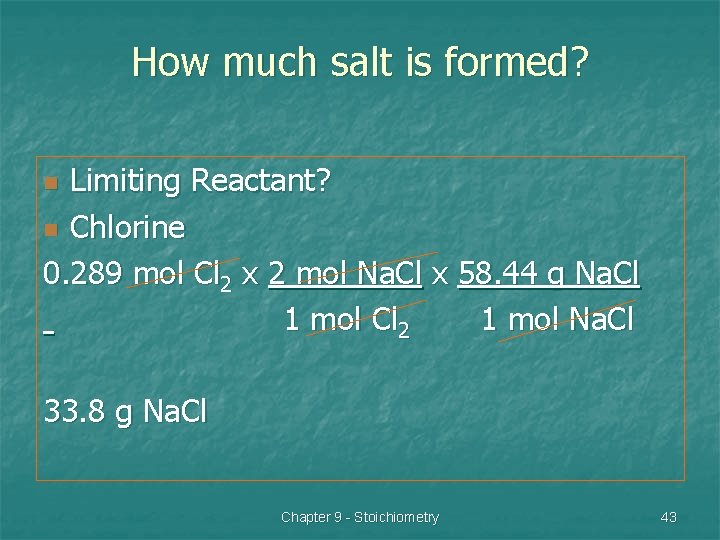
Cl 2 + 2 Na. Cl If 20. 5 g of chlorine is reacted with 20. 5 g of sodium, which is the limiting reactant? 20. 5 g Cl 2 x 1 mol Cl 2 = 0. 289 mol 70. 9 g 20. 5 g Na x 1 mol Na = 0. 892 mol 22. 99 g Cl 2 : 0. 289 ÷ 1 = 0. 289 Na : 0. 892 ÷ 2 = 0. 445 n Chapter 9 - Stoichiometry 42

How much salt is formed? Limiting Reactant? n Chlorine 0. 289 mol Cl 2 x 2 mol Na. Cl x 58. 44 g Na. Cl 1 mol Cl 2 1 mol Na. Cl n 33. 8 g Na. Cl Chapter 9 - Stoichiometry 43

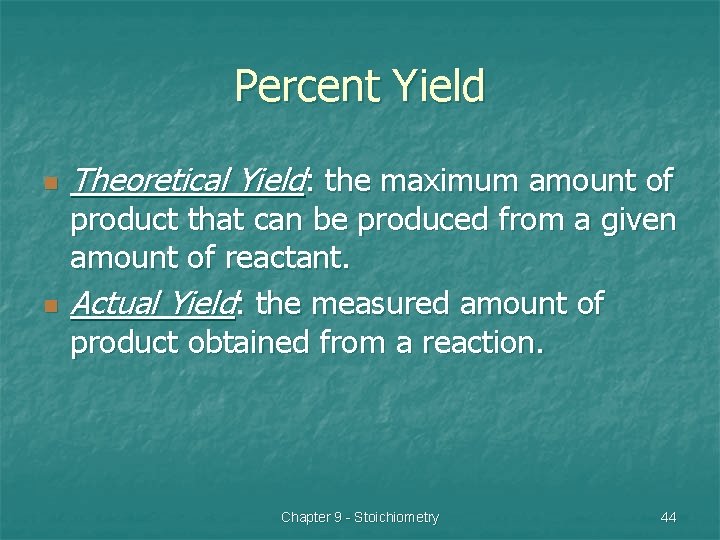
Percent Yield n n Theoretical Yield: the maximum amount of product that can be produced from a given amount of reactant. Actual Yield: the measured amount of product obtained from a reaction. Chapter 9 - Stoichiometry 44

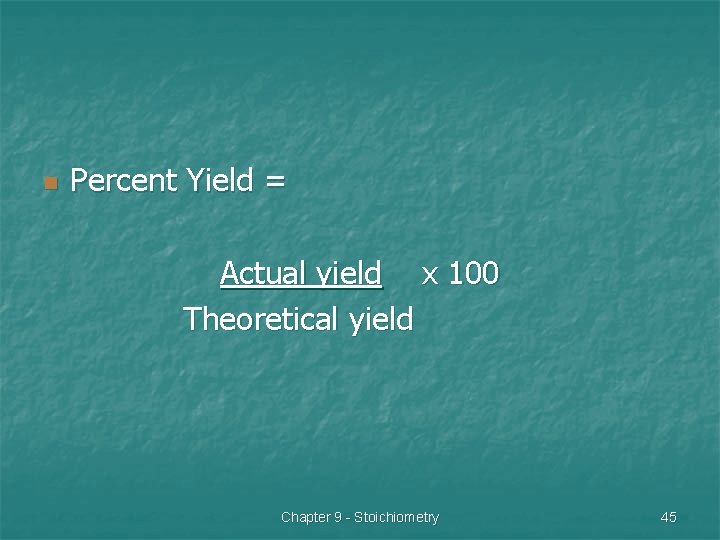
n Percent Yield = Actual yield x 100 Theoretical yield Chapter 9 - Stoichiometry 45

Example 1 n n Chlorobenzene is used in the production of many important chemicals, such as aspirin, dyes, and disinfectants. One industrial method of preparing chlorobenzene is to react benzene, with chlorine, as represented in the following reaction: C 6 H 6 (l) + Cl 2 (g) C 6 H 5 Cl (s) + HCl (g) Chapter 9 - Stoichiometry 46

n n When 36. 8 g of C 6 H 6 react with an excess of Cl 2, the actual yield of C 6 H 5 Cl is 38. 8 g. What is the percent yield of C 6 H 5 Cl? C 6 H 6 (l) + Cl 2 (g) C 6 H 5 Cl (s) + HCl (g) Chapter 9 - Stoichiometry 47

Step 1: n n Do a mass-mass calculation to find theoretical yield. Answer: 53. 0 g Chapter 9 - Stoichiometry 48

Step 2: n Use percent yield equation: 38. 8 g x 100 53. 0 g n Answer: 73. 2% Chapter 9 - Stoichiometry 49

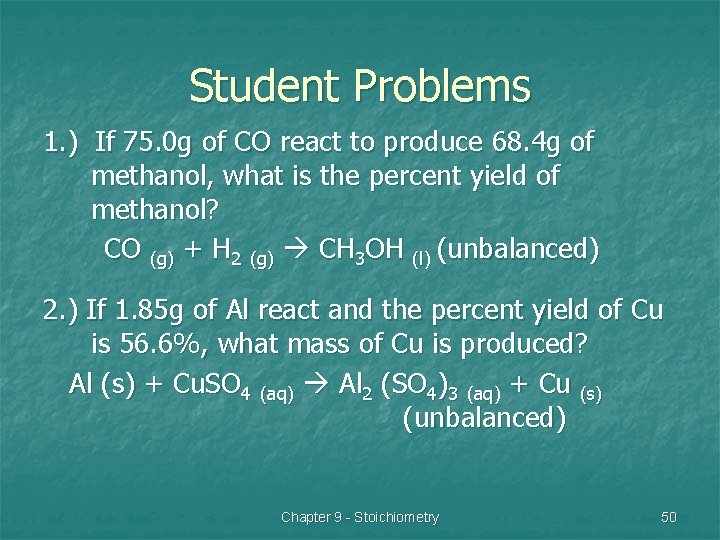
Student Problems 1. ) If 75. 0 g of CO react to produce 68. 4 g of methanol, what is the percent yield of methanol? CO (g) + H 2 (g) CH 3 OH (l) (unbalanced) 2. ) If 1. 85 g of Al react and the percent yield of Cu is 56. 6%, what mass of Cu is produced? Al (s) + Cu. SO 4 (aq) Al 2 (SO 4)3 (aq) + Cu (s) (unbalanced) Chapter 9 - Stoichiometry 50

Answers 1. 2. 79. 8% 3. 70 g Chapter 9 - Stoichiometry 51