Stoichiometry Chapter 12 Chemistry Making Chocolate Chip Cookies




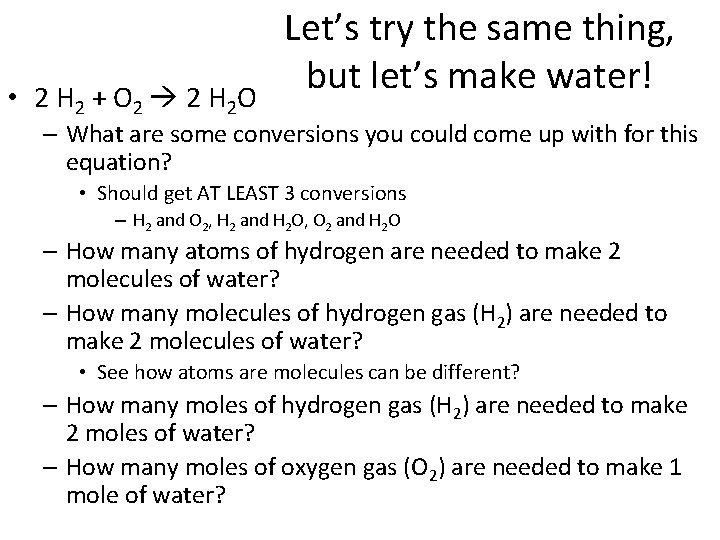



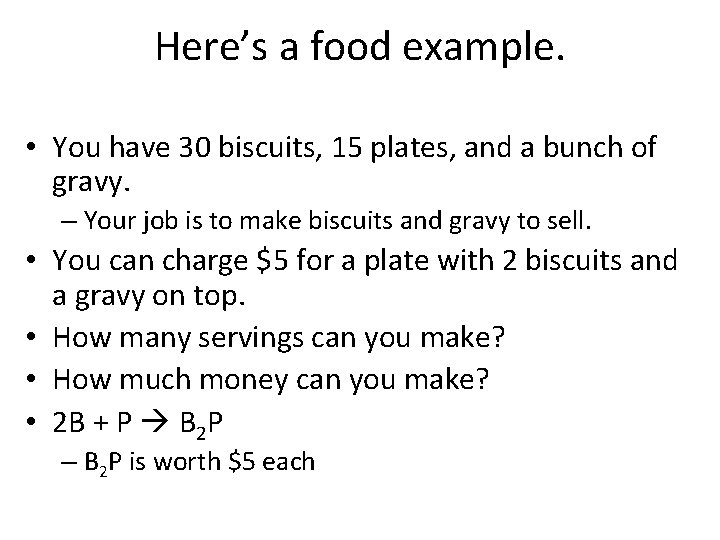
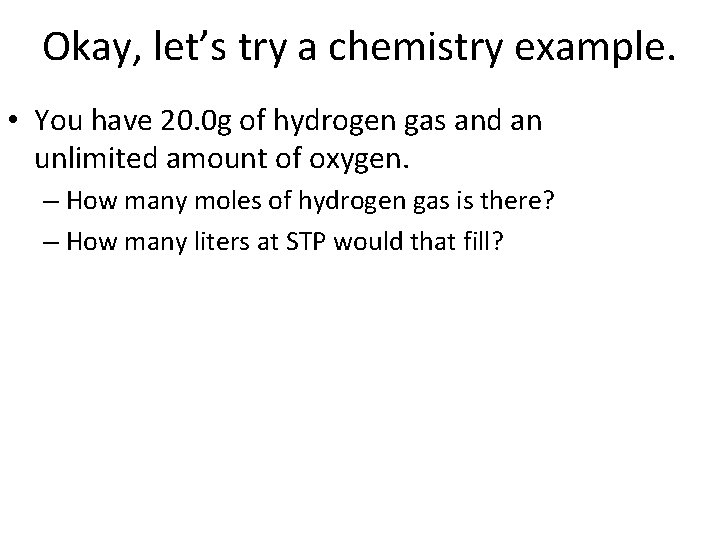
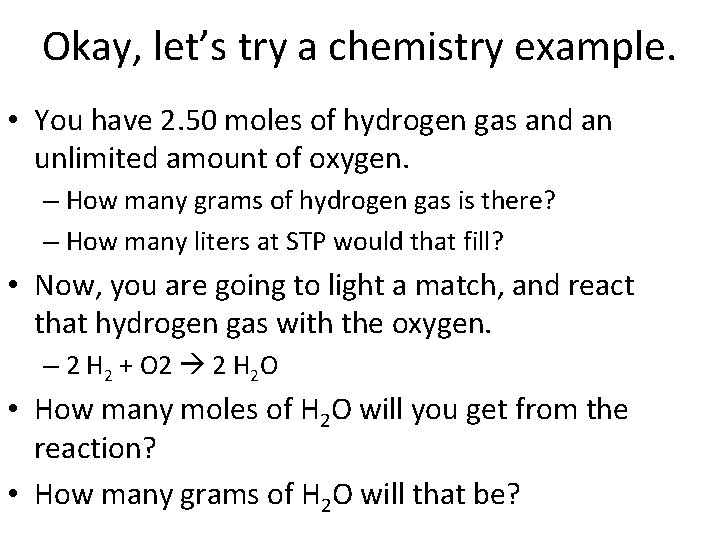


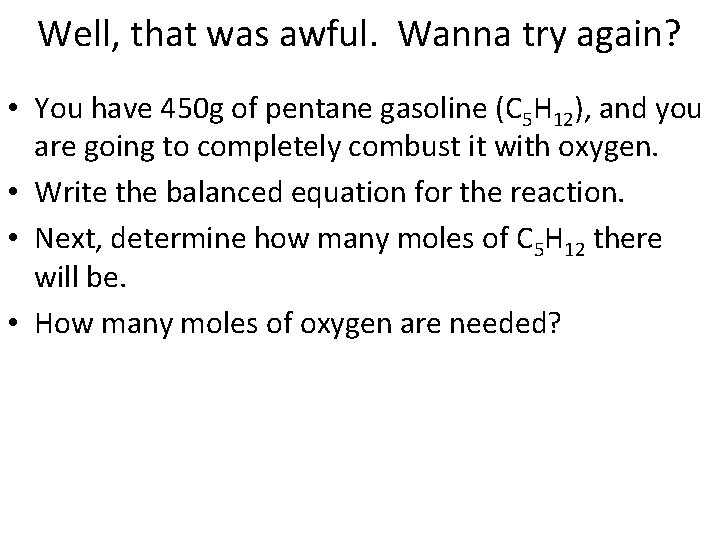










- Slides: 34

Stoichiometry Chapter 12 Chemistry

Making Chocolate Chip Cookies • This recipe makes 20 cookies. • How much oatmeal is needed to make 10 cookies? • How many eggs are needed to make 100 cookies? • How many cups of flour are needed to make 1 cookie?

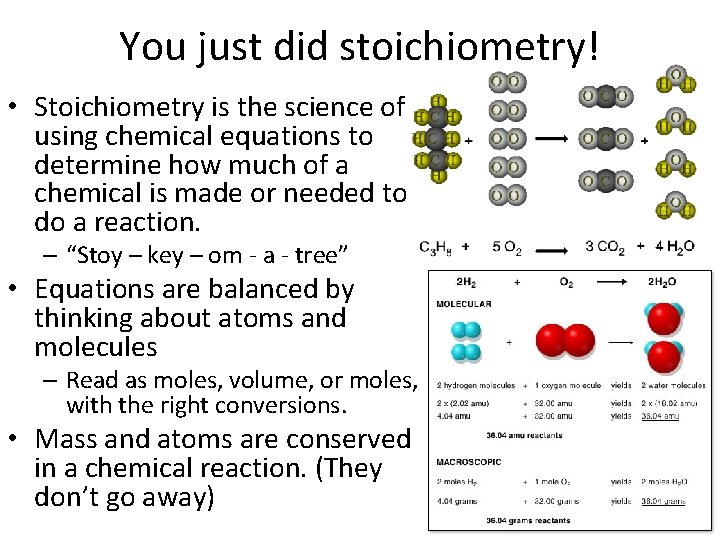
You just did stoichiometry! • Stoichiometry is the science of using chemical equations to determine how much of a chemical is made or needed to do a reaction. – “Stoy – key – om - a - tree” • Equations are balanced by thinking about atoms and molecules – Read as moles, volume, or moles, with the right conversions. • Mass and atoms are conserved in a chemical reaction. (They don’t go away)

Remember conversions? • A conversion is just a ratio where the top is equal to the bottom. – Ex) 1 km = 0. 61 mi, so the conversion would be: 1 km 0. 61 mi – Which then can be multiplied by any number to convert miles to km, or km to miles. – Try this one: How many km/hr is 60 mi/hr?

Okay, one more cooking example. (I was hungry, okay? ) • Two cans of water (mass of 200 g each) plus one can of concentrated lemonade (mass of 250 g) gives you two liters of lemonade. • What is the total mass of the lemonade? – Come up with conversions you could use from this information. – How many cans of concentrated lemonade and water would you need to make 1 liter of lemonade? – What would be the mass of water, if you wanted to make 40 L of lemonade?

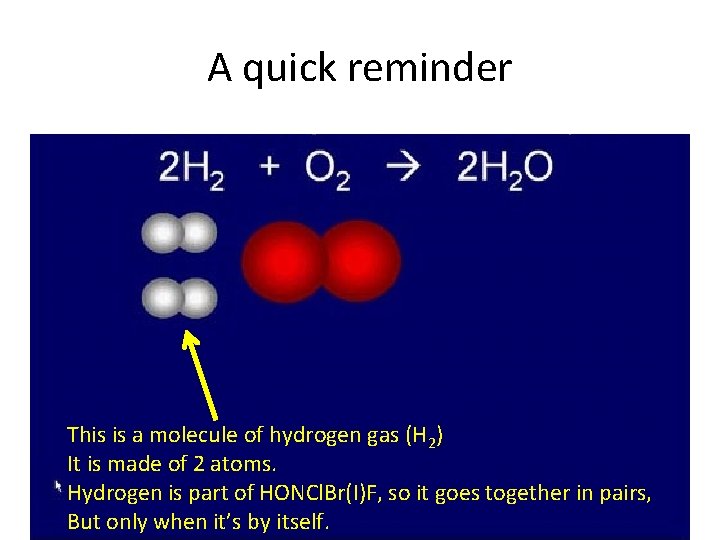
A quick reminder This is a molecule of hydrogen gas (H 2) It is made of 2 atoms. Hydrogen is part of HONCl. Br(I)F, so it goes together in pairs, But only when it’s by itself.
• 2 H 2 + O 2 2 H 2 O Let’s try the same thing, but let’s make water! – What are some conversions you could come up with for this equation? • Should get AT LEAST 3 conversions – H 2 and O 2, H 2 and H 2 O, O 2 and H 2 O – How many atoms of hydrogen are needed to make 2 molecules of water? – How many molecules of hydrogen gas (H 2) are needed to make 2 molecules of water? • See how atoms are molecules can be different? – How many moles of hydrogen gas (H 2) are needed to make 2 moles of water? – How many moles of oxygen gas (O 2) are needed to make 1 mole of water?

Let’s try one on our own. • 2 Al + 3 Cl 2 2 Al. Cl 3 – Write at least 3 conversions then solve… – How many moles of chlorine gas (Cl 2) are needed to make 1 mole of Al. Cl 3? – How many moles of aluminum chloride are produced from 10 moles of aluminum? – How many ATOMS of chlorine are needed to make 30 molecules of aluminum chloride?

Let’s do some quick review. • If you have the reaction of 3. 78 moles of C 2 H 2 combusted in this reaction: – 2 C 2 H 2 + 5 O 2 4 CO 2 + 2 H 2 O – How many moles of carbon dioxide would it produce? – How many moles of water would it produce?

Just a little review… • How many liters are in 2. 68 moles of O 2? • How many moles are in 76 grams of SO 3? • How many grams are in 126. 0 L of N 2?

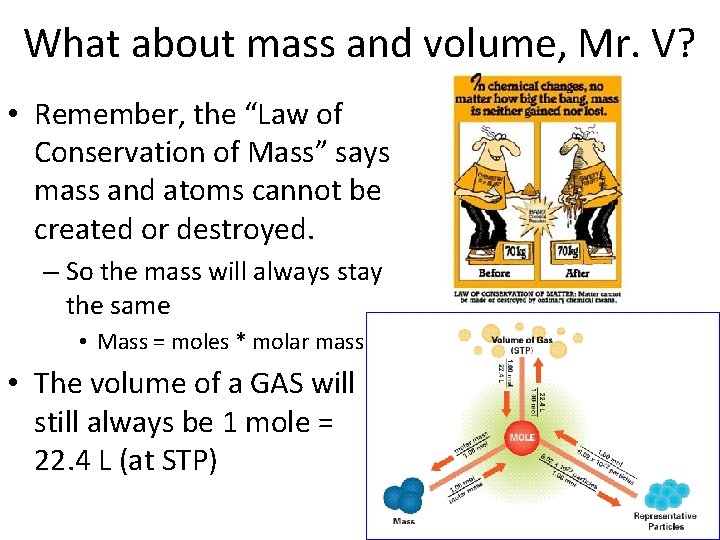
What about mass and volume, Mr. V? • Remember, the “Law of Conservation of Mass” says mass and atoms cannot be created or destroyed. – So the mass will always stay the same • Mass = moles * molar mass • The volume of a GAS will still always be 1 mole = 22. 4 L (at STP)
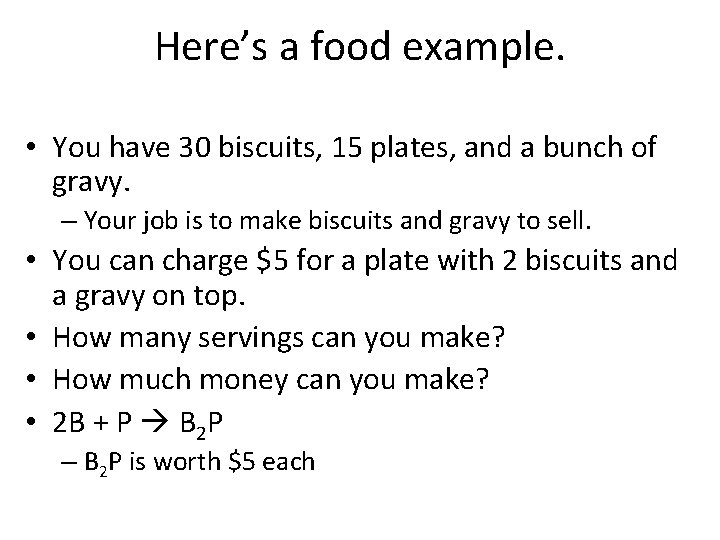
Here’s a food example. • You have 30 biscuits, 15 plates, and a bunch of gravy. – Your job is to make biscuits and gravy to sell. • You can charge $5 for a plate with 2 biscuits and a gravy on top. • How many servings can you make? • How much money can you make? • 2 B + P B 2 P – B 2 P is worth $5 each
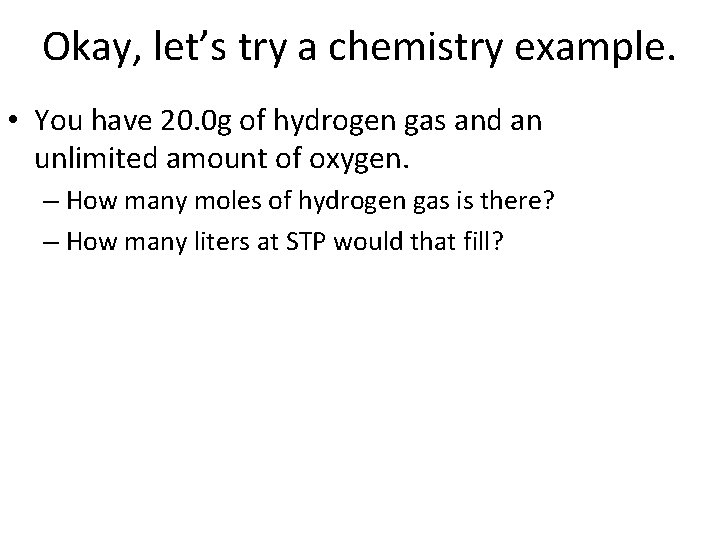
Okay, let’s try a chemistry example. • You have 20. 0 g of hydrogen gas and an unlimited amount of oxygen. – How many moles of hydrogen gas is there? – How many liters at STP would that fill?
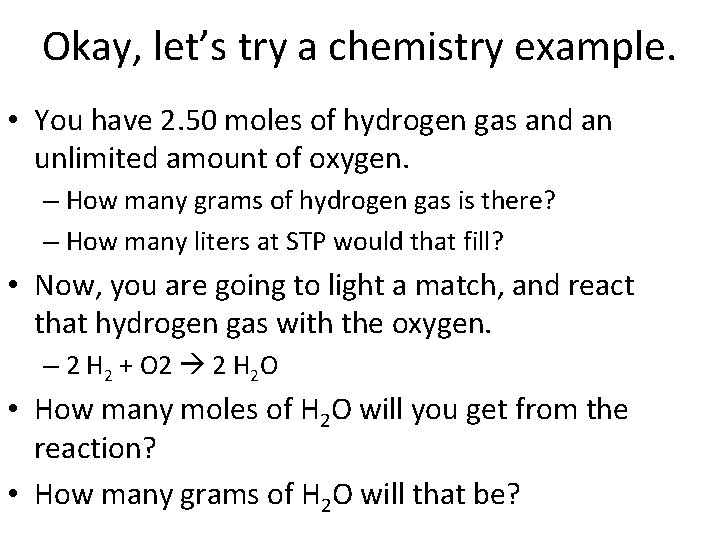
Okay, let’s try a chemistry example. • You have 2. 50 moles of hydrogen gas and an unlimited amount of oxygen. – How many grams of hydrogen gas is there? – How many liters at STP would that fill? • Now, you are going to light a match, and react that hydrogen gas with the oxygen. – 2 H 2 + O 2 2 H 2 O • How many moles of H 2 O will you get from the reaction? • How many grams of H 2 O will that be?

Well, that was awful. Wanna try again? • You have 450 g of pentane gasoline (C 5 H 12), and you are going to completely combust it with oxygen. • Write the balanced equation for the reaction.

Well, that was awful. Wanna try again? • You have 450 g of pentane gasoline (C 5 H 12), and you are going to completely combust it with oxygen. • Write the balanced equation for the reaction. • Next, determine how many moles of C 5 H 12 there will be.
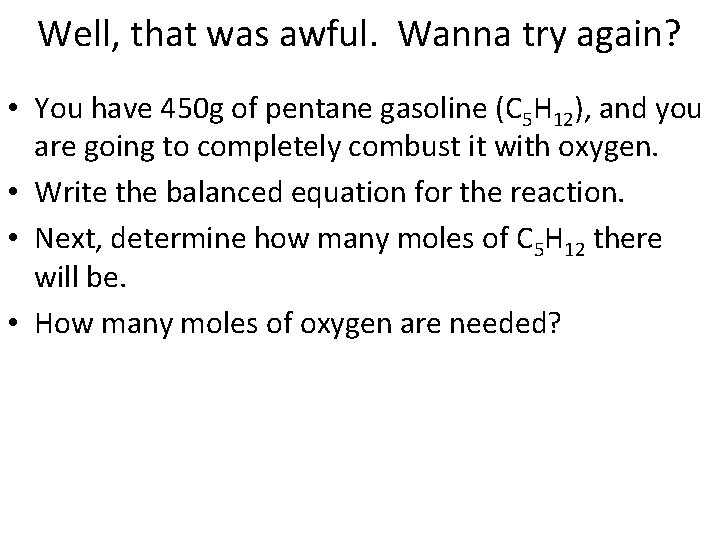
Well, that was awful. Wanna try again? • You have 450 g of pentane gasoline (C 5 H 12), and you are going to completely combust it with oxygen. • Write the balanced equation for the reaction. • Next, determine how many moles of C 5 H 12 there will be. • How many moles of oxygen are needed?

Well, that was awful. Wanna try again? • You have 450 g of pentane gasoline (C 5 H 12), and you are going to completely combust it with oxygen. • Write the balanced equation for the reaction. • Next, determine how many moles of C 5 H 12 there will be. • How many moles of oxygen are needed? • How many moles of water and carbon dioxide will be made?
Well, that was awful. Wanna try again? • You have 450 g of pentane gasoline (C 5 H 12), and you are going to completely combust it with oxygen. • Write the balanced equation for the reaction. • Next, determine how many moles of C 5 H 12 there will be. • How many moles of oxygen are needed? • How many moles of water and carbon dioxide will be made? • And how many grams of water and carbon dioxide is that? How many liters of carbon dioxide is it?

Okay, now let’s try some on our own. (This is what your 12 B Quiz is going to look like. ) • You are going to react sodium with chlorine gas. 1. Write a balanced reaction. 2. You have a 2. 00 g piece of sodium. How many moles is that? 3. How many grams and liters of chlorine are needed? 4. How many moles of Na. Cl will be formed? 5. How many grams will this be?

Limiting Reagents. • I like food. • You’re out camping and you have 15 packets of Top Ramen. • Each packet needs 500 m. L of water. • You only brought 6000 m. L of water. • How many packets of Top Ramen can you make?

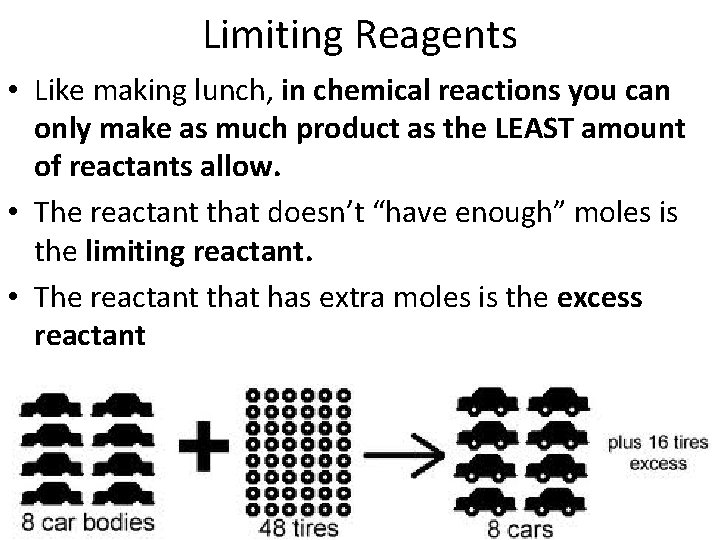
Limiting Reagents • Like making lunch, in chemical reactions you can only make as much product as the LEAST amount of reactants allow. • The reactant that doesn’t “have enough” moles is the limiting reactant. • The reactant that has extra moles is the excess reactant

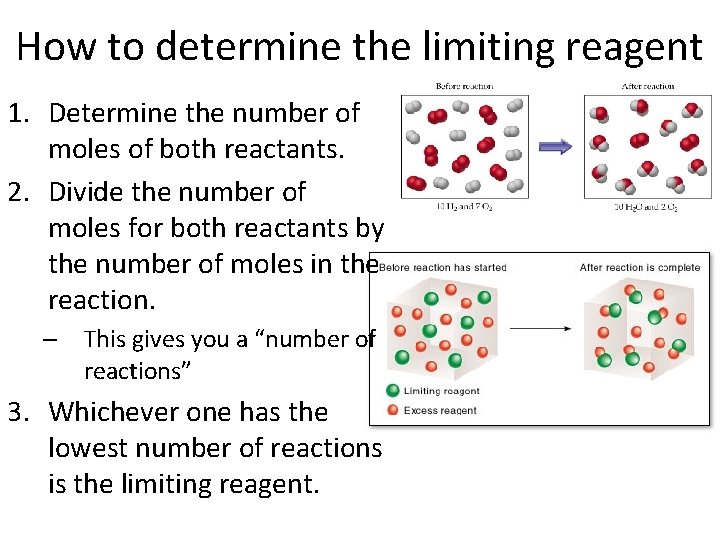
How to determine the limiting reagent 1. Determine the number of moles of both reactants. 2. Divide the number of moles for both reactants by the number of moles in the reaction. – This gives you a “number of reactions” 3. Whichever one has the lowest number of reactions is the limiting reagent.

Okay, let’s try one together. • Reaction: – CH 4 + 2 O 2 CO 2 + 2 H 2 O • You have 7. 0 moles of CH 4 and 10. 0 moles of O 2. • How many reactions can each reactant do? • What is the limiting reactant?

Not bad. Now, let’s try one without moles. • You have 30. 0 grams of Na metal. • You have 24. 5 L of chlorine gas (Cl 2) • They react like this: – 2 Na + Cl 2 2 Na. Cl • • How many moles of each do you have? How many reactions of each can each do? What is the limiting reactant? What is the excess reactant?

How about this one, on your own. • If you mix potassium metal with water, you get a huge reaction. – One scientist who worked with potassium called it “evil” • The reaction is 2 K + 2 H 2 O 2 KOH + H 2 • You have 20. 0 grams of water, and 40 grams of water. • How many moles of each? • How many reactions of each? • What is the limiting reactant?

What do we do with this? (And why is it so important? ) • To calculate products, we use only the limiting reactant’s moles. • So, once you’ve figured out the limiting reactant, you “start over” and you can figure out the number of moles of each product! – You can ignore the excess reactant. • Limiting reactants is just one more small step added to stoichiometry.
Let’s try an example. • You have 309 g of nitrogen gas (N 2) and 45. 1 g of hydrogen gas. – N 2 + 3 H 2 2 NH 3 • What is the limiting reagent? – How many moles of each? – How many reactions of each?
Let’s try an example. • You have 309 g of nitrogen gas (N 2) and 45. 1 g of hydrogen gas. – N 2 + 3 H 2 2 NH 3 • What is the limiting reagent? • How many moles of NH 3 would be produced? • How many grams of NH 3 are produced?

Okay, try this one on your own. • You have 2. 00 L of F 2 and 0. 700 g of Al. – 2 Al + 3 F 2 2 Al. F 3 • • How many moles of fluorine gas is there? How many moles of aluminum is used? How many reaction can be done? What is the limiting reactant?

Okay, try this one on your own. • You have 2. 00 L of F 2 and 0. 700 g of Al. – 2 Al + 3 F 2 2 Al. F 3 How many moles of fluorine gas is there? How many moles of aluminum is used? How many reaction can be done? What is the limiting reactant? How many moles of Aluminum fluoride would be produced? • How many grams of aluminum fluoride would be produced? • • •

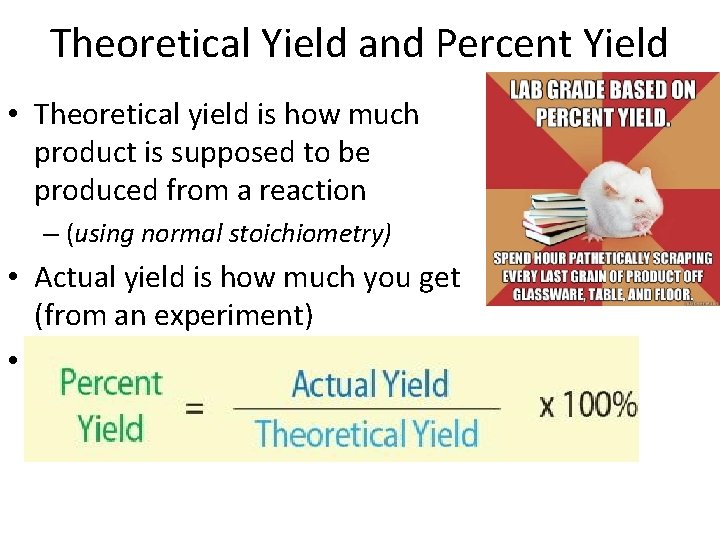
Theoretical Yield and Percent Yield • Theoretical yield is how much product is supposed to be produced from a reaction – (using normal stoichiometry) • Actual yield is how much you get (from an experiment) • Percent yield = actual yield * 100 theoretical yield

Let’s try one. • You have a reaction where your theoretical yield is 40. 0 g, but you only get 37. 2 g of product. • What is your theoretical yield?
Okay, now a big one… • You combust 37. 5 g of C 3 H 8 in an excess of oxygen, and collect 5. 3 g of water. 1. What is your theoretical yield of water? 2. What is your actual yield of water? 3. What is your percent yield?