Stoichiometry Chemical Recipes Mr Mole Cookies u When




















- Slides: 29

“Stoichiometry” Chemical Recipes Mr. Mole

Cookies!? u When baking cookies, a recipe is usually used, telling the exact amount of each ingredient. • The recipe suggests how much you need to start with to get a certain amount at the end. • If you need more, you can double or triple the amount u Thus, a recipe is much like a balanced equation in they require set ratios u Equation example 2

Cookie Ratios u Write a “recipe equation” with symbols u Solve for # cookies (product or yield): u Solve for an ingredient from ingredient (reactant): 3

Stoichiometry is… u. Greek for “measuring elements” Pronounced “stoy kee ahm uh tree” u. Defined as: calculations of the s n o i t quantities in chemical reactions, a u q e t s a based on aakbalanced equation. f e r B u. We MUST start with a balanced equation – ALWAYS! u Because you can’t make a new recipe for “chili” without the quantities/ratios in the recipe!!! 4

Mythbusters Stoich u The Mythbusters love explosions! u A good reaction or explosion requires a balanced chemical recipe what chemists call a balanced reaction. u Visible Shockwave: https: //www. youtube. com/watch? v=f. Gi 0 w 7 yc. RKk u What if you don’t have enough oxygen or add too much gasoline? ? ? -> fizzles out. u In order to know how much of each “ingredient” you need stoichiometry. Myth: Can a sewer explode and kill blow off the manhole covers? Low quality video here Skip 7: 05 -11: 43 skip 16 Siberian Methane build up : https: //m. youtube. com/watch? v=FM 0 hcz. FNDZI 5

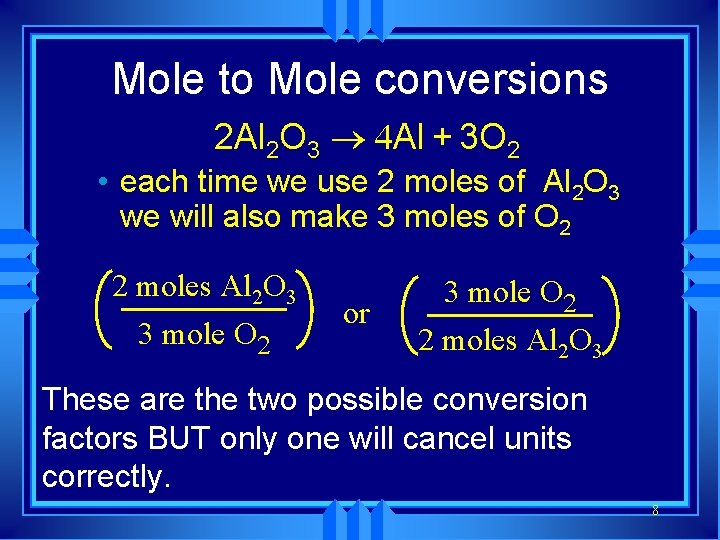
How do we convert from one compound to another? u. We use the coefficient in the balanced equation. 2 Al 2 O 3 ® 4 Al + 3 O 2 u This is called the u mole ratio: ratio of one compound to another in a balanced equation obtained by using the coefficient. u Aluminum to oxygen? u Aluminum oxide to aluminum?

Stoic Example: Mole to Mole conversion Y N u. How many moles of Oo are A f 2 e t h t n u d o n i f m n a n produced when 3. 34 moles of r a u e t c h t d u n o w y a o , s n l n k a o i c u t Al 2 Oif 3 ydecompose? i c o m a e e r h c e w ! r h o s t e r N h e n O e® t t i i l o l r a 2 Al 4 Al + 3 O c o i h l t 2 3 2 m o L e m L h c s A f m o a r 3 mol O 2 t g n u o o Al Oit t 3. 34 = 5. 01 mol O 2 ammol 2 3 2 mol Al 2 O 3 LABELED Conversion factor (mole ratio) from balanced equation 7

Mole to Mole conversions 2 Al 2 O 3 ® 4 Al + 3 O 2 • each time we use 2 moles of Al 2 O 3 we will also make 3 moles of O 2 2 moles Al 2 O 3 3 mole O 2 or 3 mole O 2 2 moles Al 2 O 3 These are the two possible conversion factors BUT only one will cancel units correctly. 8

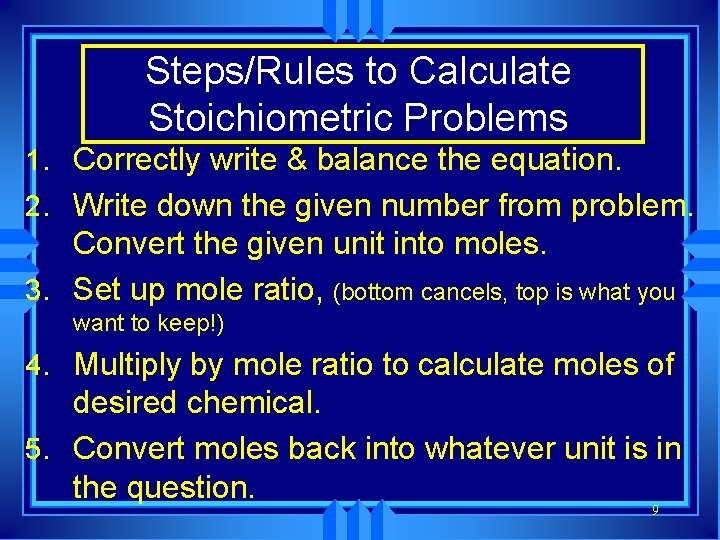
Steps/Rules to Calculate Stoichiometric Problems 1. Correctly write & balance the equation. 2. Write down the given number from problem. Convert the given unit into moles. 3. Set up mole ratio, (bottom cancels, top is what you want to keep!) 4. Multiply by mole ratio to calculate moles of desired chemical. 5. Convert moles back into whatever unit is in the question. 9

YOU Practice: 2 C 2 H 2 + 5 O 2 ® 4 CO 2 + 2 H 2 O • If 3. 84 moles of C 2 H 2 are burned, how many moles of O 2 are needed? 10

YOU Practice: 2 C 2 H 2 + 5 O 2 ® 4 CO 2 + 2 H 2 O • If 2. 47 moles of C 2 H 2 are burned, how many moles of CO 2 are formed? 11

How do you get good at this? Mol-Mol WS 12

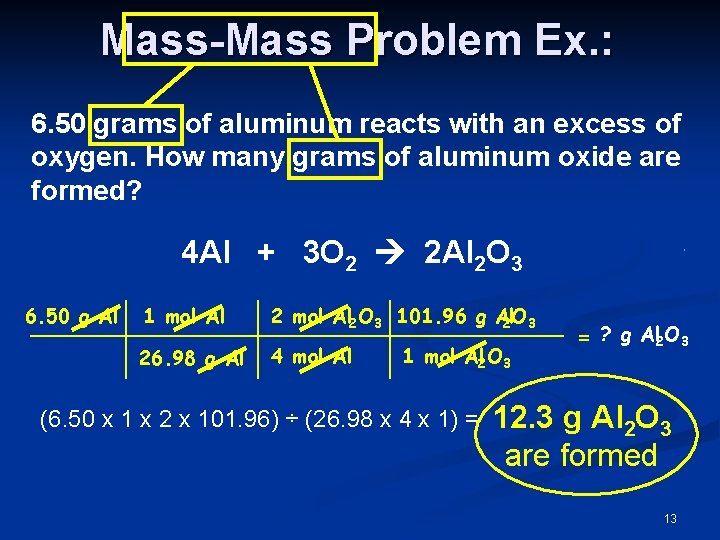
Mass-Mass Problem Ex. : 6. 50 grams of aluminum reacts with an excess of oxygen. How many grams of aluminum oxide are formed? 4 Al + 3 O 2 2 Al 2 O 3 6. 50 g Al 1 mol Al 2 O 3 101. 96 g Al 2 O 3 26. 98 g Al 4 mol Al 1 mol Al 2 O 3 (6. 50 x 1 x 2 x 101. 96) ÷ (26. 98 x 4 x 1) = = ? g Al 2 O 3 12. 3 g Al 2 O 3 are formed 13

YOU Practice u. If 10. 1 g of Fe are added to a solution of Copper (II) Sulfate, how many grams of solid copper would form? 2 Fe + 3 Cu. SO 4 ® Fe 2(SO 4)3 + 3 Cu Answer = 17. 2 g Cu 14

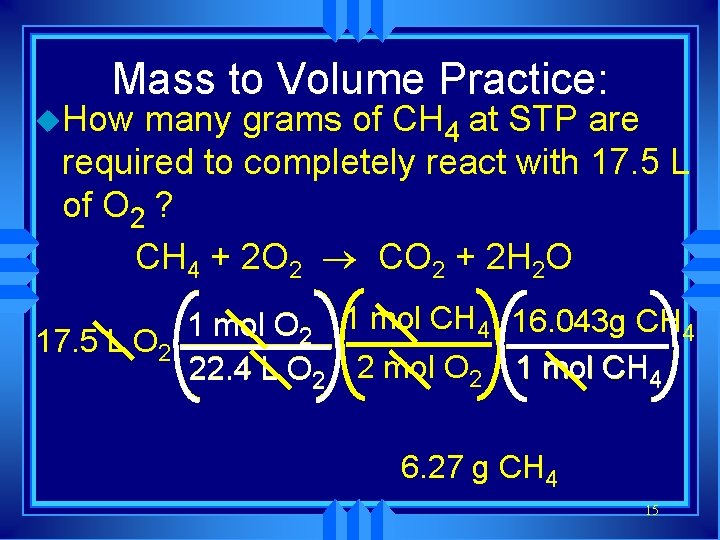
Mass to Volume Practice: u. How many grams of CH 4 at STP are required to completely react with 17. 5 L of O 2 ? CH 4 + 2 O 2 ® CO 2 + 2 H 2 O 1 mol O 2 1 mol CH 4 16. 043 g CH 4 17. 5 L O 2 22. 4 L O 2 2 mol O 2 1 mol CH 4 6. 27 g CH 4 15

u. The “Limiting” Reagents recipe for my world famous pancakes is the following: u 1 cup water + ½ cup Aunt Jemima mix 10 pancakes u. If I have 1 gallon (16 cups) of water and ¼ cup of mix, how many pancakes can I make? u 160 according to the water. BUT u. Since the mix runs out… only 5! 16

“Limiting” Reagent u. The limiting reagent is the reactant you run out of first. u. The excess reagent is the one you have left over. u. The limiting reagent determines how much product you can make 17

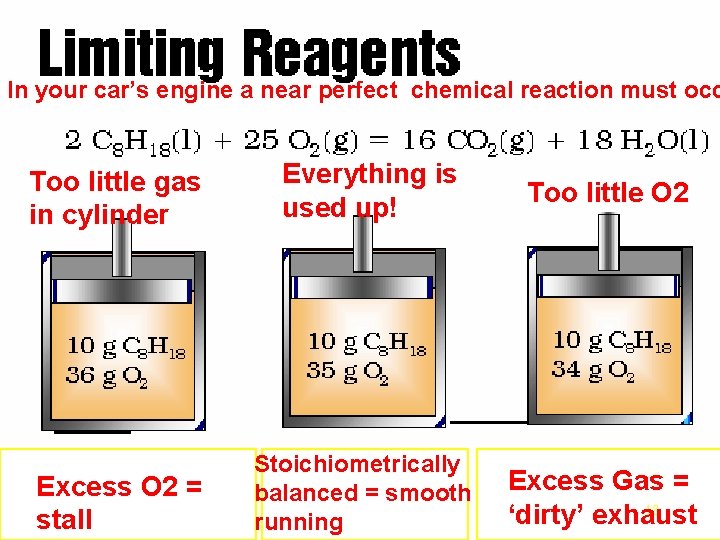
Limiting Reagents - Combustion In your car’s engine a near perfect chemical reaction must occ Too little gas in cylinder Everything is used up! Too little O 2 Excess O 2 = stall Stoichiometrically balanced = smooth running Excess Gas = 18 ‘dirty’ exhaust

How do you find out are working on a limited reactant problem? u. You can recognize limiting reagent problems because they will give you 2 amounts for chemical reactants u. The chemical that makes the least amount of product is the “limiting reagent or reactant”. 19

How to Solve a Limiting Reactant Problem 1. 2. Recognize it as a limiting reactant (2 amounts of chemical reactants given) Write a balanced formula 3. Do two stoichiometry problems, one for each reactant you are given SOLVE FOR SAME PRODUCT. 4. The problem with the smaller product had the “limiting reactant” The problem with the larger product had the “excess reactant” Using limited reactant solve for the excess reactant and subtract to get how much excess remained. 5. 6. 20

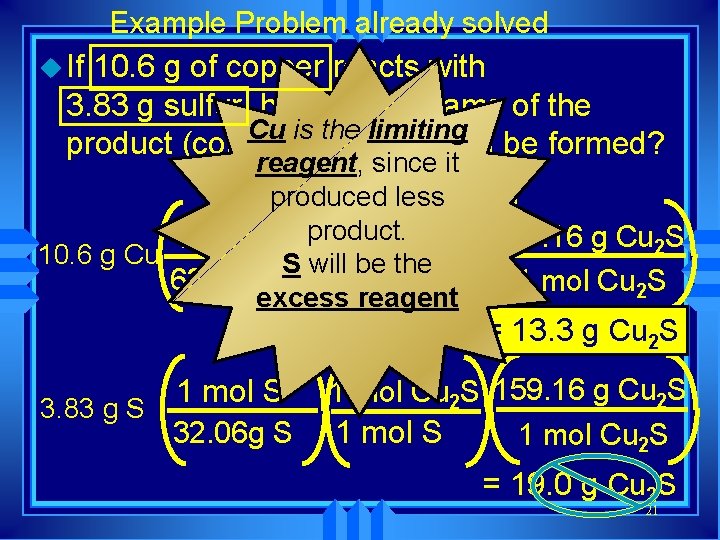
Example Problem already solved u If 10. 6 g of copper reacts with 3. 83 g sulfur, how many grams of the Cu is the limiting product (copper (I) sulfide) will be formed? reagent, since it 2 Cuproduced + S ® Cu less 2 S product. 1 mol Cu 2 S 159. 16 g Cu 2 S 1 mol Cu 10. 6 g Cu S will be the 63. 55 g Cu 2 mol Cu 1 mol Cu 2 S excess reagent 1 mol S 3. 83 g S 32. 06 g S = 13. 3 g Cu 2 S 1 mol Cu 2 S 159. 16 g Cu 2 S 1 mol Cu 2 S = 19. 0 g Cu 2 S 21

Cu. SO 4 is limited, so Cu = 20. 6 g much excess reactant will remain? Excess = 4. 47 grams PR u. How AC u the TI C E p 1 7 More Limiting Reactant Practice u. If 10. 3 g of aluminum are reacted with 51. 7 g of Cu. SO 4 how much copper (grams) will be produced? 2 Al + 3 Cu. SO 4 → 3 Cu + Al 2(SO 4)3 22

The Concept of: A little different type of yield than you will have in Driver’s Education class. That means “ give the right of way” 23

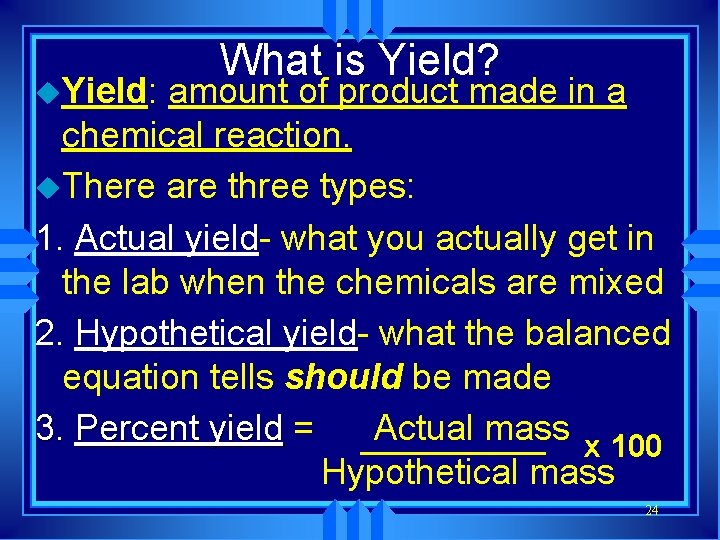
u. Yield: What is Yield? amount of product made in a chemical reaction. u. There are three types: 1. Actual yield- what you actually get in the lab when the chemicals are mixed 2. Hypothetical yield- what the balanced equation tells should be made 3. Percent yield = Actual mass x 100 Hypothetical mass 24

u. Percent Details on Yield yield tells us how “efficient” a reaction is or how well it works. u. Percent yield can not be bigger than 100 %. u. Hypothetical yield will always be larger than actual yield! • Why? Due to impure reactants; competing side reactions; Heat added or lost, loss of product in filtering or transferring between containers; measuring human error is NEVER Accepted. 25

u 6. 78 Percent Yield Example: g of copper is produced when 3. 92 g of Al are reacted with excess copper (II) sulfate. What is the Percent Yield? 2 Al + 3 Cu. SO 4 ® Al 2(SO 4)3 + 3 Cu u. What is the actual yield ? = 6. 78 g Cu u. What is the hypothetical yield? MUST = 13. 84837588 g BE OBTAINED WITH STOICH Cu u. Percent yield? = 49. 0 % 26

27

XAvogadro told us: u. Equal volumes of gas, at the same temperature and pressure contain the same number of particles. u. Moles are numbers of particles u. You can treat reactions as if they happen liters at a time, as long as you keep the temperature and pressure the same. 1 mole = 22. 4 L @ STP 28

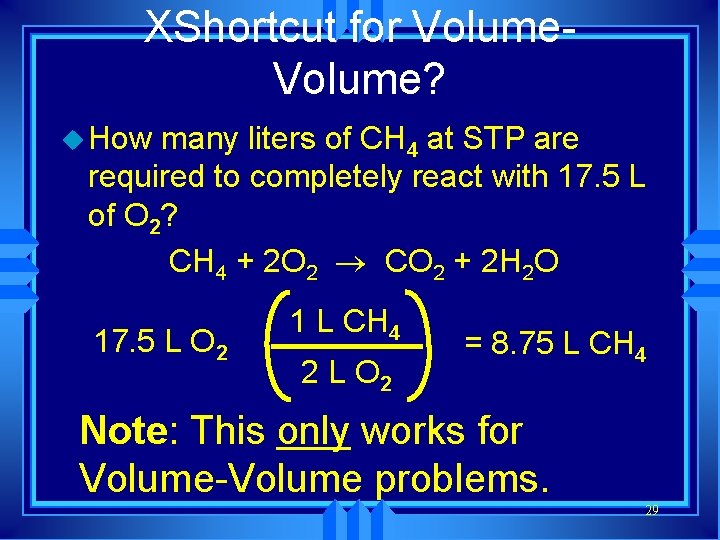
XShortcut for Volume? u How many liters of CH 4 at STP are required to completely react with 17. 5 L of O 2? CH 4 + 2 O 2 ® CO 2 + 2 H 2 O 17. 5 L O 2 1 L CH 4 2 L O 2 = 8. 75 L CH 4 Note: This only works for Volume-Volume problems. 29
 Stoichiometry: mole-mole problems
Stoichiometry: mole-mole problems Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Phosphorus and oxygen equation
Phosphorus and oxygen equation Stoichiometry mole-mole
Stoichiometry mole-mole Cookie stoichiometry
Cookie stoichiometry Mole cookies chemistry
Mole cookies chemistry What are voles
What are voles Mol island
Mol island Stoichiometry mole island diagram
Stoichiometry mole island diagram Lesson 92 mole tunnel stoichiometry
Lesson 92 mole tunnel stoichiometry Mass to moles formula
Mass to moles formula Mole-mass-volume relationships
Mole-mass-volume relationships Mole-mole factor
Mole-mole factor Baking cookies chemical or physical change
Baking cookies chemical or physical change Chemical accounting stoichiometry
Chemical accounting stoichiometry Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Stoichiometry map for chemical reactions
Stoichiometry map for chemical reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Chemical accounting stoichiometry
Chemical accounting stoichiometry In a chemical reaction stoichiometry refers to
In a chemical reaction stoichiometry refers to Chemical rxns/balancing equ./stoichiometry
Chemical rxns/balancing equ./stoichiometry Mole relationships in chemical equations
Mole relationships in chemical equations Unit chemical quantities the mole 1 step
Unit chemical quantities the mole 1 step Docusign api recipes
Docusign api recipes Adjusting recipes worksheet answers
Adjusting recipes worksheet answers Objectives of a standard recipe
Objectives of a standard recipe Colitis diet
Colitis diet Extemporaneous compounding worksheet
Extemporaneous compounding worksheet How to make fake injuries
How to make fake injuries Wash the dishes in passive voice
Wash the dishes in passive voice